39 limiting reagent and percent yield worksheet answer key
Answers: Limiting Reagent Worksheet #1. 1. Balanced equation: C3H8 + 5 O2 -----> 3 CO2 + 4 H2O. a) O2
Limiting reagent calculations are performed in the same manner as the stoichiometric equations on worksheet 11. Step 2 determine the moles of co. Step 3 do a limiting reagent test. Limiting reagents and percentage yield worksheet 1. However with a limiting. Step 4 using the limiting reagent find the moles of i2produced.
limiting reactant and percent yield practice problems answers limiting reactant and percent yield practice worksheet answer key limiting reagent & percent yield practice worksheet answer key limiting reagent and percent yield practice problems answers. Post Navigation.

Limiting reagent and percent yield worksheet answer key
Moles molecules and grams worksheet answer key with work. Limiting reactant and percent yield worksheet answer key. Al worksheets pre writing worksheets for 3 year olds limiting reagent worksheet answer key grade 8 maths geometry worksheets 5th grade capacity worksheets 2nd grade dragon worksheets tayutay worksheet grade 6 sixth.
You can learn 6 pages limiting reagent and percent yield worksheet 2 analysis in Doc format. Limiting reagent worksheet 2. A If 20 mol of HF is combined with 45 mol of SiO2 which is the limiting reactant. Limiting reagent worksheet 2 answer key. Limiting reagents and percentage yield worksheet 1.
Limiting reactant and percent yield worksheet answer key or 24 fresh stoichiometry limiting reagent worksheet percent recovery denotes the quantity of the original substance retained after the conclusion of the reaction. Ii what percentage yield of iodine was produced. However with a limiting.
Limiting reagent and percent yield worksheet answer key.
Answers to Worksheet #14 Limiting Reagents A Limiting Reagent is the reactant that is completely used up in a reaction. This reagent is the one that determines the amount of product formed. Limiting reagent calculations are performed in the same manner as the stoichiometric equations on Worksheet #11. However, with a limiting
Limiting Reagents and Percentage Yield Review Worksheet 1. Consider the reaction I 2 O 5 (g) + 5 CO(g) -----> 5 CO 2 (g) + I 2 (g) a) 80.0 grams of iodine(V) oxide, I 2 O 5, reacts with 28.0 grams of carbon monoxide, CO. Determine the mass of iodine I 2, which could be produced? b) If, in the above situation, only 0.160 moles, of iodine, I 2
Practice Problems: Limiting Reagents (Answer Key) Take the reaction: NH 3 + O 2 NO + H 2 O. In an experiment, 3.25 g of NH 3 are allowed to react with 3.50 g of O 2. a. Which reactant is the limiting reagent? ... What is the percent yield for the conversion of ethanol to acetic acid if O 2 is in excess? 30.3%.
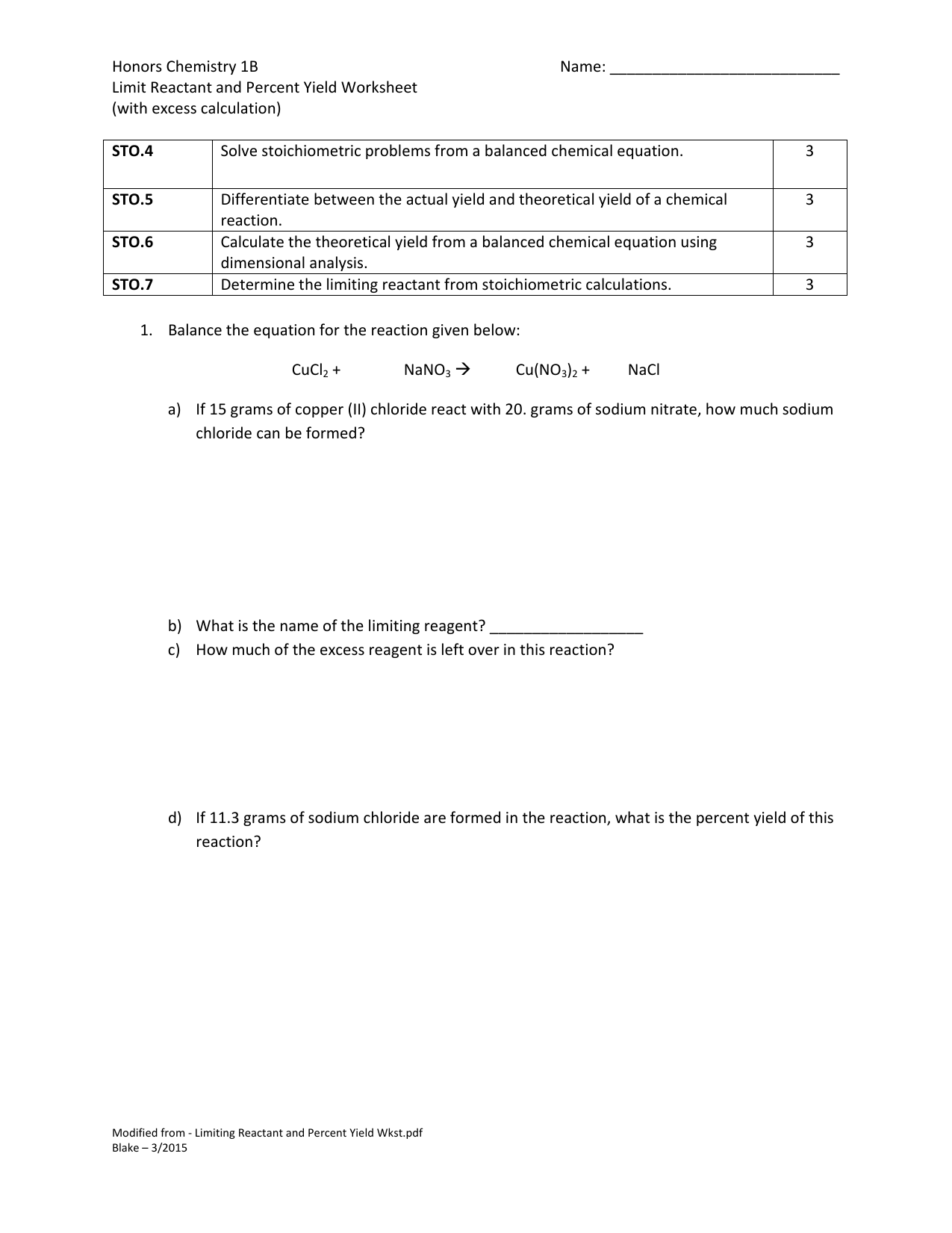
What was the percent yield? Zn + HCl ( ZnCl2 Limiting Reagent Worksheet -KEY. All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed. 1) Write the balanced equation for the reaction given above:
View notes limiting reagents and percentage yield worksheet answers 1 from chem 214 at old dominion university. The substance that has the smallest answer is the limiting reagent. Limiting Reactant and Percent Yield Worksheet Answer Key or 24 Fresh Stoichiometry Limiting Reagent Worksheet.
Limiting reactant and percent yield worksheet answer key or 24 fresh stoichiometry limiting reagent worksheet percent recovery denotes the quantity of the original substance retained after the conclusion of the reaction. Via a list of easy to do precisely how to s to many well researched examples this specific grouping is stuffed with an array ...
what is my percent yield? (18.5 / 17.2) x 100% = 108% c) Is the answer from problem b) reasonable? Explain. No. Any yield over 100% is a violation of the Law of conservation of mass. d) If I do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of sodium phosphate will I make?
Limiting reagents and percentage yield worksheet. Worksheet limiting reactants answer key grade 10 types of reactions worksheet two step equations worksheet step by step. Limiting reactant worksheet answer key free printables. Answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction.
Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C 3H 8 + O 2-----> CO 2 + H 2O a) If you start with 14.8 g of C 3H 8 and 3.44 g of O 2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H 2O produced
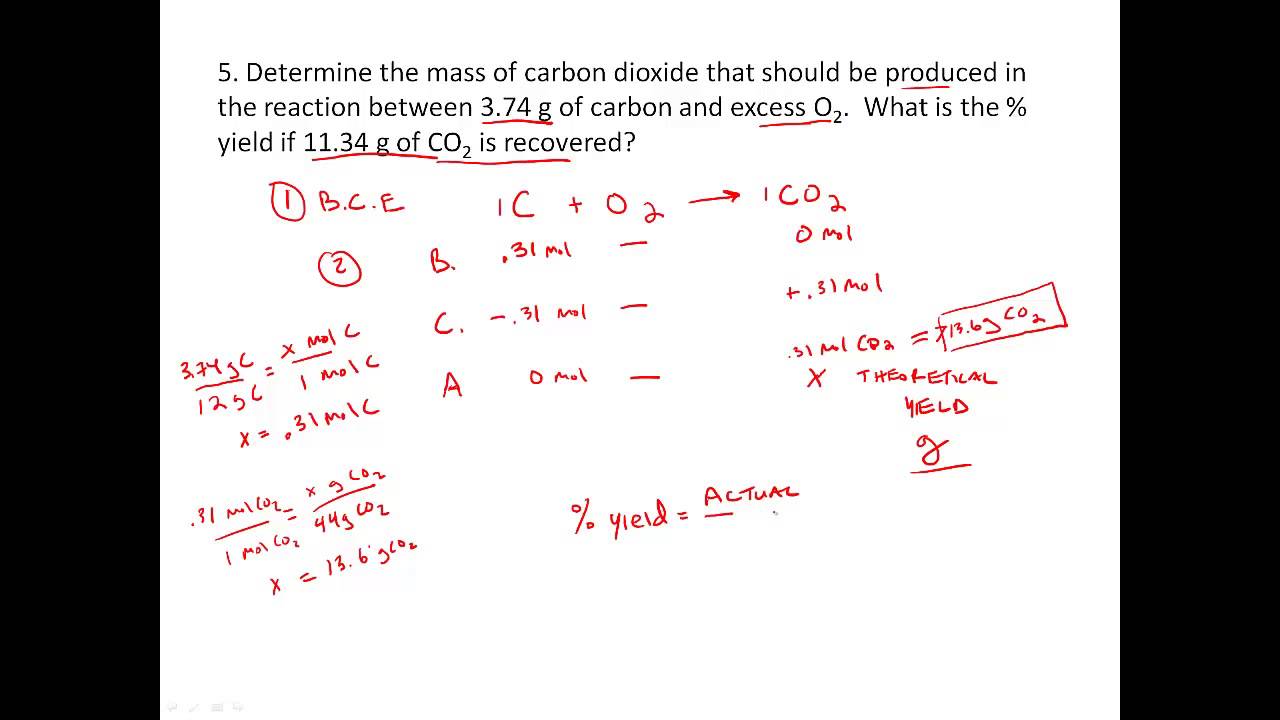
Actual yield was 3.5 x 103 g CH 3 OH. In order to calculate % yield, we need the theoretical yield (max amount of CH 3 OH that could be formed using the amount of reactants given). Since CO is said to be in excess, 5.0 x 103 g H 2 (all) reacts: 342 33 23 23 1 mol H 1 mol CH OH made 32.04 g CH OH 5.0 x 10 g H x x x =3.973 x 10 g CH OH
Created Date: 1/27/2016 7:41:57 AM
Limiting Reactants and Percent Yield Worksheet by MJ 15 $1.50 Word Document File This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. A complete answer key is provided at the end.
Worksheet 14 3 Answers to Worksheet 14 Limiting Reagents A Limiting Reagent is the reactant that is completely used up in a reaction. Limiting reagents and percentage yield worksheet. C6H5Br HBr a. Both of the following give you the same answer. What is the limiting reactant. Limiting Reagent Worksheet Answers.
Chemistry 12 3 Limiting Reagent And Percent Yield Chemistry Pure Products Purity. Stoichiometry Quiz Persuasive Writing Prompts Handwriting Worksheets For Kindergarten Algebra Worksheets. Stoichiometry Limiting Reactants Mastery Mats 16 Task Cards Step By Step Task Cards Cards Task. Percent Yield And Stoichiometry Notes And Practice Problems ...
Using Limiting Reagents; Percentage Yield; Answer Key: Percentage Yield . 1. For the balanced equation shown below, if the reaction of 90.6 grams of CO produces 36.7 grams of C 3 H 8, what is the percent yield? 3CO+7H 2 =>C 3 H 8 +3H 2 O C= 12 O= 16
CO is limiting Determine the mass of iodine I 2, which could be produced? 50.7 g b) If, in the above situation, only 0.160 moles, of iodine, I 2 was produced. i) what mass of iodine was produced? 40.6 g ii) what percentage yield of iodine was produced. 80.1% 2. Zinc and sulphur react to form zinc sulphide according to the equation.
5) If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Limiting Reagent Worksheet All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed.
Practice some actual yield and percentage problems below. 1. For the balanced equation shown below, if the reaction of 40.8 grams of C6H6O3 produces a 39.0% yield, how many grams of H2O would be produced ? C6H6O3+6O2=>6CO2+3H2O 2.
W.LimitingReagentsandPercentYield.HW1.ANSWERKEY - Name_ANSWER KEY Date Period Limiting Reagent Percent Yield Practice Worksheet 1 When copper(II
The limiting reagent is the reagent that determines the quantity of product that may be formed using a response. Often, it's essential to recognize the limiting reagent in an issue. Limiting Reactant and Percent Yield Worksheet Answer Key with Percent Yield Worksheet 1 Kidz Activities.
Limiting Reagent Worksheet -KEY 1) Write the balanced equation for the reaction given above: CuCl 2 + 2 NaNO 3 Cu(NO 3) 2 + 2 NaCl 2) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride
Limiting reactant and percent yield worksheet answers. Determine the mass of iodine i 2 which could be produced. Using co as the limiting reagent a reaction of 28 0 grams of co will produce 50 76 grams of iodine. Determine the mass of iodine i2 which could be produced. Limiting reagents and percentage yield review worksheet 1.
The Results for Limiting Reactant Percent Yield Answers Key. Structure Worksheet. Limiting Reactant and Percent Yield Worksheet







![Percent Yield Worksheet [gen50z9k1klo]](https://idoc.pub/img/crop/300x300/34wm92ydj8l7.jpg)

























0 Response to "39 limiting reagent and percent yield worksheet answer key"
Post a Comment