41 ph of salt solutions worksheet answers
Solutions. Answers to Chemistry End of Chapter Exercises. 2. (a) oxidation-reduction (addition); (b) acid-base (neutralization); (c) oxidation-reduction (combustion) 4. It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction. 6. A salt solution can be deemed acidic, basic, or neutral depending on the pH, which affects the ions that it interacts with. Learn about salt solutions by understanding salt at a molecular level ...
Electrolytes are aqueous solutions that conduct electricity. Acids, bases and salts (ionic compounds) are electrolytes. Nonelectrolytes are aqueous solutions that do not conduct electricity. The solutes used to form nonelectrolytes are covalently bonded. Classify the following as conductors or nonconductors by writing C or N next to each. Name

Ph of salt solutions worksheet answers
https://www.iitutor.comSalts can be classified as basic, acidic or neutral. Basic salts contain the conjugate base (X-) of a weak acid(HX) that hydrolyses in... Rinse the pH meter probe with distilled water before every reading. Use solutions with known pH values, see Table 2, to make sure the pH meter is accurately calibrated. Make sure the pH meter probe is properly submerged in the solution before taking a reading. Bibliography Bibliography Read Online Ph Of Salt Solutions Worksheet Answers chapter 1 solution, biology cst released test questions 2013 answers, audi a4 b6 repair manual, holt environmental science chapter 13 review answers, ditch witch 410sx tech manual, engineering drawing design 4th edition, international business by ricky w griffin and michael w pustay free ...
Ph of salt solutions worksheet answers. Ex 3) Calculate the pH of a 0.150M solution of ammonia. The K b of ammonia is 1.80x10-5. Ex 4) Calculate the pH of a 0.227M solution of hydrocyanic acid (HCN). The K a=6.2×10−10. SALT SOLUTION (HYDROLYSIS) To find the pH of a salt solution, first determine which ion within the salt will undergo hydrolysis. In other 2. Calibrate the pH probe for acidic solution and measure the pH of each HCl solution. 3. Prepare 50.0 mL of the following solutions by serial dilution from the stock NaOH solution: 0.10 M NaOH, 0.010 M NaOH, and 0.0010 M NaOH. 4. Calibrate the pH probe for basic solution and measure the pH of each NaOH solution. Procedure 2: Weak Acid and Base 1. • 15.3 pH and the Autoionization of Water • 15.4 Calculations Involving pH, K a, and K b • 15.5 Polyprotic Acids • 15.6 pH of Salt Solutions • 15.7 The Common-Ion Effect • 15.8 pH Buffers • 15.9 pH Indicators and Acid-Base Titrations • 15.10 Solubility Equilibria pH of Salt Solutions 1. Sample Problem: Salt Hydrolysis. If we dissolve NaF in water, we get the following equilibrium: The pH of the resulting solution can be determined if the of the fluoride ion is known. 20.0 g of sodium fluoride is dissolve in enough water to make 500.0 mL of solution. Calculate the pH of the solution. The of the fluoride ion is 1.4 × 10 −11 .
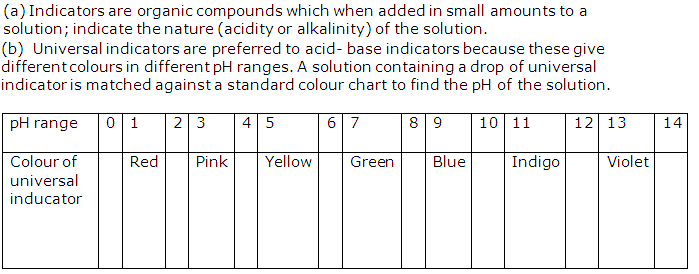
Double click in center of graph Jun 24, 2013 · Virtual Chem Lab 6-2 & 6-3 Anonymous CHM/110 June 16, 2014 Zamir Deen Virtual Chem Lab 6-2 & 6-3 Ranking Salt Solutions by pH 6-2: Ranking Salt Solutions by pH In this assignment you will be asked to rank aqueous solutions of acids, bases, and salts in order of increasing pH. . Sep 17, 2021 · The salt in this example is known as the solute, the thing dissolved in the water. The more salt, or solute, you add to the water, the higher the concentration of solute you have in your solution. solutions normally show acidic or basic natures, considering the strength of ions hydrolyzing in water. Different structures of salts will produce various pH ranges in an aqueous solution. Salt is defined as the formed ionic compound derived from a neutralization reaction between an acid and a base. The pH scale indicates the concentration of hydrogen and hydroxide ions in solutions. Acid pH range: 0-7 Examples: (vary) coffee, lemon juice Base pH range: 7-14 more OH- than H+ Examples: (vary) bleach, drain cleaner Water equal amounts of OH- and H+ Examples: ten; Changes in pH can disrupt cells’ chemical reactions and homeostasis.
salt electrolytes L--water auc\tc If pH is less than 7, the substance is If the pH is 7, the substance is If pH is more than 7, the substance is Most substances are (acid, base, neutral) 10. I can identify acids, bases, conjugate acids, and conjugate bases in a Bronsted-Lowry reaction. B) Answer # 6, 7, 8 pages 588, 589 (see p.801 for # 7) C) Investigation 8.3.1 (see page 827) • choose one obviously acidic salt choose one obviously basic salt choose one additional salt that is either amphiprotic, or which contains ions which both affect pH. The pH Scale: Calculating the pH of a Solution 9:49 Acid-Base Buffers: Calculating the pH of a Buffered Solution 7:25 Go to Behaviors & Properties of Solutions Acid-Base Properties of Salt Solutions Worksheet 1. What is a salt? ... How is pH affected by the concentration of a solution? 5. ... Sort answers by oldest.1 answer · 0 votes: Base salt (1) What is salt and how it is formed ? salt is chemically neutral compound formed by the neutralisation reaction of acid and base . example ...
C- Solutions 2, 5 and 6 are bases, solution 3 is an acid, solution 1 is a salt and solution 4 can not be classified D- Solution 3 is a base, solutions 2, 5 and 6 are acids and solutions 1 and 4 are salts 8. Identify the following pH as either acid, base or salt: pH 6 pH 8 pH 14 pH 2 pH 11 pH 7 9.
ph of salt solutions worksheet answers is available in our digital library an online access to it is set as public so you can download it instantly. Our digital library hosts in multiple countries, allowing you to get the most less latency time to download any of our books like this one. ...
Measuring pH of Common Substances Worksheet Answers 1. Name all the items that are acidic according to your tests. Vinegar, lemon juice, tomato/apple juice 2. What characteristics do all these items have in common? Tastes sour, turns litmus paper red, turns cabbage juice red 3. Name all the items that are basic according to your tests.
Worksheet: Acid base problems - AP level Problems 1 - 10 Problem #1: Calculate the pH of the solution that results when 40.0 mL of 0.100 M NH 3 is: a) Diluted with 20.0 mL of distilled water. b) Mixed with 20.0 mL of 0.200 M HCl solution.
Calculating pH and pOH worksheet W 335 Everett Community College Tutoring Center Student Support Services Program 1) What is the pH of a 0.0235 M HCl solution? 2) What is the pOH of a 0.0235 M HCl solution? 3) What is the pH of a 6.50 x 10-3 M KOH solution? (Hint: this is a basic solution - concentration is of OH-)
Calculating the pH of a Salt Solution. To calculate the pH of a salt solution one needs to know the concentration of the salt solution, whether the salt is an acidic, basic, or neutral salt, the equation for the interaction of the ion with the water, the equilibrium expression for this interaction and the K a or K b value.
Calculating pH for Titration Solutions: Strong Acid/Strong Base A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 M of a strong base NaOH the titration curve is shown in Figure 1. Calculate the pH at these volumes of added base solution: (a) 0.00 mL (b) 12.50 mL (c) 25.00 mL (d) 37.50 mL. Solution
Calibrate the pH meter using standard pH buffer solutions such as pH = 4.0, 7.0, and 10.0. Place about 10 mL of solution in a small beaker and determine the pH by immersing the pH probe into the solution. Record the pH reading on the report form, rinse the pH electrode using a wash bottle, and measure the remaining solutions in the same manner.
Examples of calculating pH of 0.25 M solution of sodium acetate, and calculating the pH of 0.050 M solution of ammonium chloride. Watch the next lesson: http...
Part A: pH solution and hydrolysis salt 1. 5 ml of unboiled distilled water was added into each separate six test tubes. 2. Three drops of different indicators was added into each test tube. The indicator used were Methyl orange, Methyl red, Bromothymol blue, Phenolphthalein, Alizarin yellow-R, Phenol red. 3.
Transcribed image text: Lab 9: Studying the pH of Strong Acid, Weak Acid, Salt, and Buffer Solutions Name Section Date Data Sheet 1 1. Preparing HCI Solutions and Determining phi Calculate these concentration of HCI, M measured pH theoretical pH 1.0 x 10-1 1.0 x 10-2 1.0 x 10-3 1.0 x 10-4 1.08 2.06 2.95 3.95 Calculate ONE of these IL Preparing HC W202 Solutions and Determining pH concentration ...
Activities This "Acid and Base" (doc) worksheet from Mr. Guch's Calvacade of Chemistry uses the Brønsted-Lowry theory of acids and bases. Mr. Guch provides these worksheets for practice with pH: "pH Practice" (doc), "pH Calcuations" (pdf), and "pH Review Problems (pdf). All Mr. Guch's worksheets include answers. Dr. Greenbowe has a "Solutions of Acids, Bases, and Salts ...
Hint: We will probably need to convert pOH to pH or find [H3O+] using [OH−] in the final stages of this problem. Answer: 11.11. Equilibrium in a Solution of a ...
Worksheet 20 - Polyprotic Acids and Salt Solutions K a Acid Base K b strong acid HNO 3, HI, HCl, etc NO 3-, I-, Cl-, etc negligible basicity 1.3 x 10-2 HSO 4-SO 4 ... Rank the following 0.1 M aqueous salt solutions in order of increasing pH. (Hint: write out the reactions of the salts + water) a) KNO 3 K 2SO 4 K 2S neutral basic (K b = 7.7x10 ...
Salt solutions may be acidic, basic, or neutral, depending on the original acid and base that formed the salt. ... What is the pH of a 0.1 mol/L solution of NaCN? See the other answers in the hydrolysis problems pdf 2. Calculate the [OH-], pOH and pH of these solutions: a) 0.50 mol/L KCN b) 0.20 mol/L NaClO . Author: Gillian Ashford Created ...
ph of salt solutions worksheet answers.pdf FREE PDF DOWNLOAD NOW!!! Source #2: ph of salt solutions worksheet answers.pdf FREE PDF DOWNLOAD gcse Index of pH scale, ACIDS, BASES, ALKALIS, SALT ...
Title: Microsoft Word - Worksheet20_Polyprotic_Salts.doc Author: Amy Created Date: 11/7/2010 6:45:28 PM
Unit 6, Lesson 08: The pH of Salt Solutions, Answers ... If not, write “no reaction” or NR. pH of ion in solution. (acidic, basic or neutral).6 pages
Finally you will use equation (3) to design and prepare a buffer of a specific pH. Procedure Part 1, pH of salt solutions: You will need a calibrated pH meter and 4 clean and dry 50 mL beakers, one containing distilled water to rinse the pH probe. Select 3 clean and dry 50 mL beakers
Chapter 15 Worksheet 4 (ws15.4) Acid-Base Properties of Salt Solution, Polyprotic. Acids. All acid-base reactions produce another acid and another base. The reaction between the stronger acid and the stronger base is called a "neutralization reaction". Neutralization reactions produce a salt (an ionic compound) and (sometimes) water.
This quiz and worksheet will measure your understanding of acidic and basic solutions. Topics that you should be familiar with include the pH level of NaCl solution and an example of a strong base ...
HYDROLYSIS OF SALTS. TUU. Sz. Namen. TTTTTL. Salt solutions may be acidic, basic or neutral, depending on the original acid and base that formed the salt.2 pages
Read Online Ph Of Salt Solutions Worksheet Answers chapter 1 solution, biology cst released test questions 2013 answers, audi a4 b6 repair manual, holt environmental science chapter 13 review answers, ditch witch 410sx tech manual, engineering drawing design 4th edition, international business by ricky w griffin and michael w pustay free ...
Rinse the pH meter probe with distilled water before every reading. Use solutions with known pH values, see Table 2, to make sure the pH meter is accurately calibrated. Make sure the pH meter probe is properly submerged in the solution before taking a reading. Bibliography Bibliography
https://www.iitutor.comSalts can be classified as basic, acidic or neutral. Basic salts contain the conjugate base (X-) of a weak acid(HX) that hydrolyses in...
























0 Response to "41 ph of salt solutions worksheet answers"
Post a Comment