40 emission spectra and energy levels worksheet
Emission Spectra - an overview | ScienceDirect Topics Emission spectra for atoms appear as a series of lines, because electrons fall from higher energy states to lower ones and emit energy as Although the discussion has concerned electronic energy levels in atoms, the electronic states in molecules are usually separated by similar energies. The emission spectrum of atomic hydrogen consists of a number of... Question 1. (a) The emission spectrum of atomic hydrogen consists of a number of discrete wavelengths. Explain how this observation leads to an understanding that there are discrete electron. Energy levels in atoms. [2]. (b) Some electron energy levels in atomic hydrogen are illustrated in Fig.
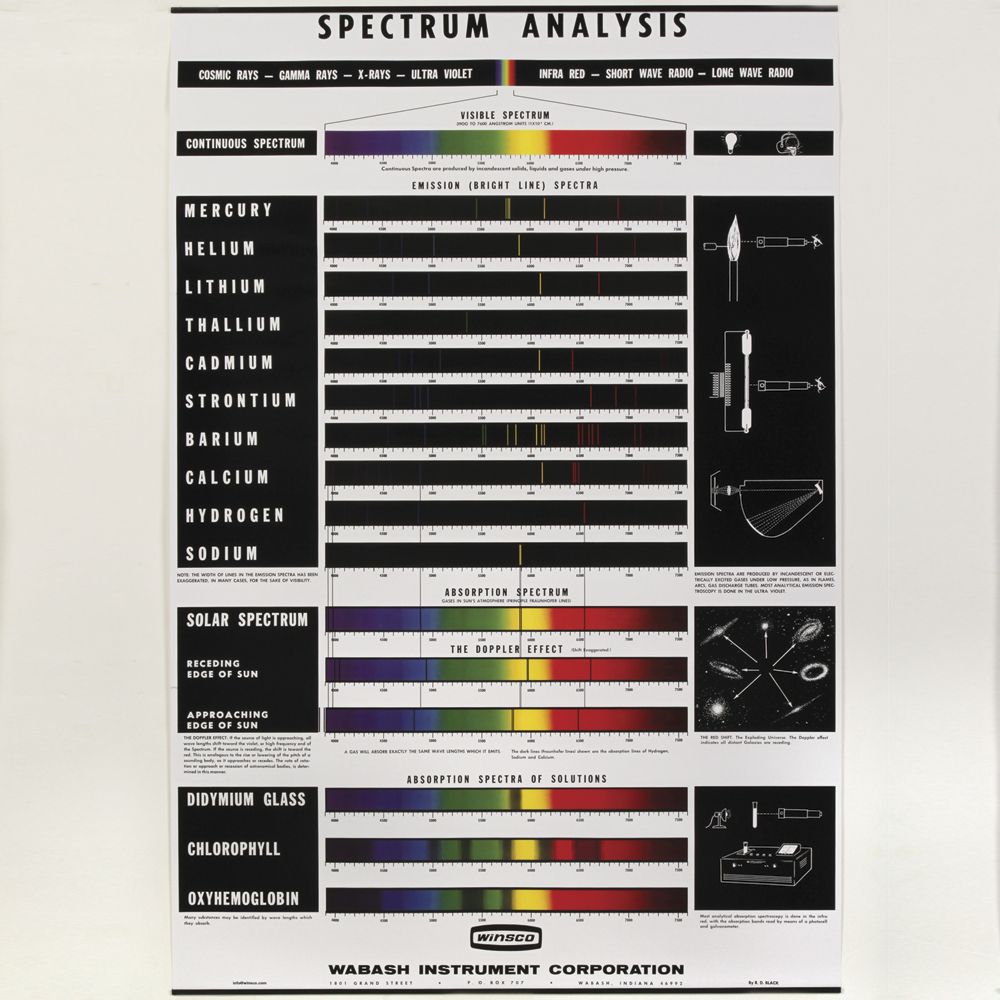
Emission Spectrum Vs. Absorption Spectrum: Know... - Science Struck The basic difference between emission and absorption spectrum is, as the name suggests, emission and absorption of light. Sounds simple? When energy in the form of light, heat, or chemical agents is given to an element, the electrons of its atoms accept the energy and go to higher energy levels.

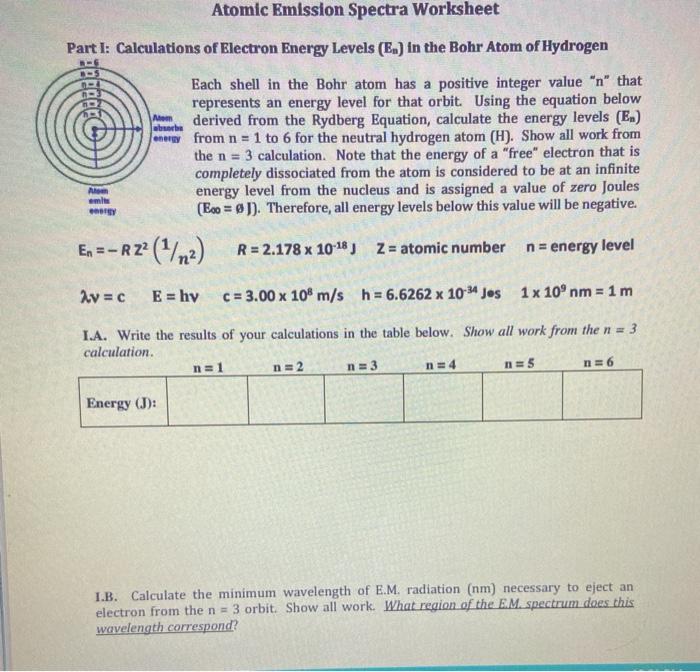
Emission spectra and energy levels worksheet
quantum mechanics - Why do atoms emit a certain colour of light? Atomic line emission and absorption spectra come from the differences between the discrete energy levels in the atom, and those are the solutions of a complex There are multiple energy levels in all atoms that electrons can occupy so there are many possible colours even for the simplest single atoms. PDF Emission spectra and energy levels worksheet A worksheet of emission spectrums and energy levels. Some of the worksheets shown are energy levels and atomic spectrum atom structure practices 1 working response lab report atomic emissions spectra emission spectra model practice work in response chapter 5 electrons atomic... PDF Microsoft Word - atomic_spectroscopy_2005.doc Note that the energy levels of the rotational states given by this expression are not equally spaced. 2/15/06. 10 Atomic and Molecular Spectroscopy. 1. Any OOIBASE spectrum saved as the scope output (with .sco extension) can be imported di-rectly into Origin worksheet.
Emission spectra and energy levels worksheet. Hydrogen energies and spectrum | Hydrogen Energy Levels The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy At left is a hydrogen spectral tube excited by a 5000 volt transformer. The three prominent hydrogen lines are shown at the right of the image through... Emission Spectra And Energy Levels — db-excel.com The essential intent behind applying Emission Spectra And Energy Levels Worksheet Answers is to provide a cement experience for students. Take advantage of time successfully and efficiently. You can focus on the example Emission Spectra And Energy Levels on this page. Emission spectrum ...to higher energy levels and the spectrum then contains many more lines for electron de-excitations to higher energy levels than the ground state. One drawback is that the total energy is lower than DC-arc excitation and the intensity of the spectral lines are lower. How to choose a suitable method? NIST: Atomic Spectra Database Lines Form Lines: All Only with transition probabilities Only with energy level classifications Only with observed wavelengths Only with diagnostics. Include diagnostics data. No JavaScript No spaces in values.
Emission Spectra and Energy Levels Worksheet Answers 3 Electromagnetic Spectrum from emission spectra and energy levels worksheet answers , source:e-education.psu.edu. Airborne pollutants come from all over the world, including smoke, dust, fumes, and other industrial chemicals. A high concentration of airborne pollutants can affect the... Difference Between Absorption and Emission Spectra | Definition... Emission Spectra: Emission spectrum can be defined as a spectrum of the electromagnetic radiation emitted by a substance. Emission Spectra: An emission spectrum is given when an excited atom obtains a lower energy level. Wavelength. Emission Spectra And Energy Levels Worksheet Answers Objectives of any Emission Spectra And Energy Levels Worksheet Answers. Worksheet needs to be include skills including drawing, analyzing, descriptive, reasoning etc. Worksheet should really be short not more than 2 pages else in the home . called as being a Workbook. Emission and Absorption Lines | Atomic energy levels and transitions Emission-line spectra. Low-density clouds of gas floating in space will emit emission lines if they are excited by energy from nearby stars. Planetary nebulae, for example, are the remnants of stars which have gently pushed their outer envelopes outwards into space. Some of them are very pretty
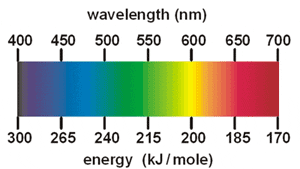
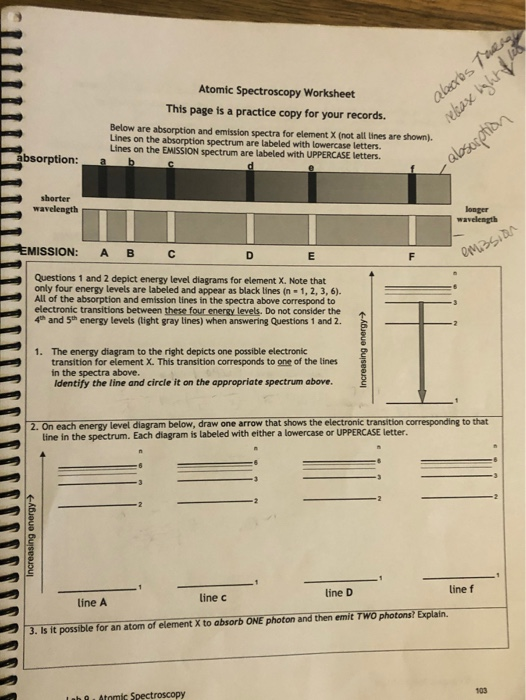
PDF Physics 30 | II. Comparison of emission and absorption spectra Emission / Bright-line Spectrum. Gases will also produce light when heated to a high temperature. In 1752 a Scottish physicist name Thomas Melvill observed the 17. What is the energy difference between the two energy levels in a sodium atom that give rise to the emission of a 589 nm photon? What is emission spectrum? - Quora An emission spectrum is what you get when you burn an element and pass the light give off by the flame through a prism. Visible Spectra of the Since the energy differences between energy levels for a certain element are constant and quantized, the emission spectrum is always the same for that... Emission Spectra And Energy Levels... - Energy Worksheet Home » Energy Worksheet » Emission Spectra And Energy Levels Worksheet Answer Key. So grab the spreadsheet & start working as soon as possible! Visit our site for more creative cards, workbooks, colouring publications, and also extra about a variety of topics, like math, chemistry, as... Absorption and Emission | Continuum, Absorption & Emission Spectra Continuum, Absorption & Emission Spectra. A given atom will absorb and emit the SAME frequencies of electromagnetic (E-M) radiation. This is called an emission spectrum (just peaks, not continuous). The E-M radiation frequencies absorbed and emitted match the allowed energy levels in the atom.
Emission spectrum - Wikipedia The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state.
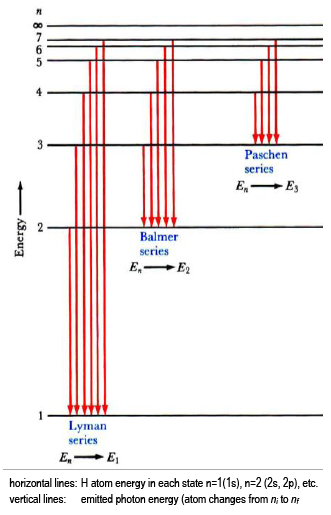
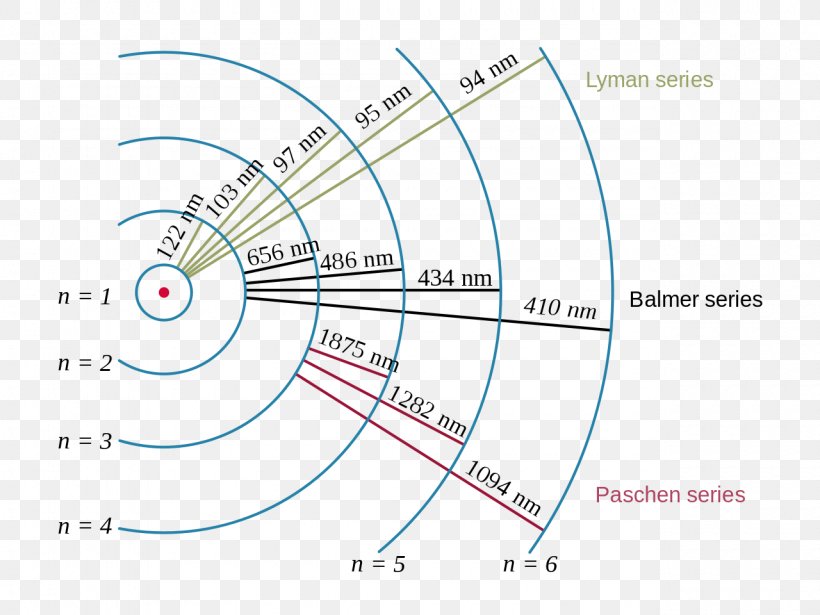
Hydrogen Spectrum - Diagram, Wavelength, Hydrogen Emission... The Energy level diagram for the Hydrogen Atom indicates the different series of lines that is observed in the Spectrum. Absorption and Emission Spectra. By placing n=1, n=2, n=3 etc in the Rydberg's equation, we can obtain the energies of the Hydrogen Electron in different stationary states.
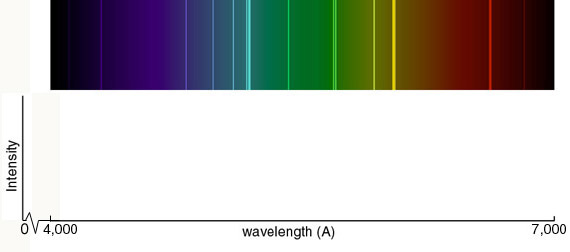
Emission Spectra and Energy Levels Worksheet Thus, emission spectra are experimental proof that electrons exist in definite, distinctive energy levels in an atom. Questions: 1. How can the difference in the brightness of spectral lines be explained? 2. According to the modern theory of the atom, where may an atom's electrons be found?
12.3 Emission and absorption spectra | Optical phenomena... | Siyavula Emission spectra (ESCQS). You have learnt previously about the structure of an atom. The electrons surrounding the atomic nucleus are arranged in a series of levels of increasing energy. Each element has a unique number of electrons in a unique configuration therefore each element has its own...
Emission and Absorption Spectra: Bohr, Line Spectrum, Examples... Emission and Absorption Spectra. The emission spectrum is the spectrum of radiation emitted by a substance that has absorbed energy. Question: An electron in a hydrogen atom transitions from energy level with n=4 to n=2. What is the wavelength of the emitted light?
atomic hydrogen emission spectrum An introduction to the atomic hydrogen emission spectrum, and how it can be used to find the ionisation energy of hydrogen. The ionisation energy per electron is therefore a measure of the distance between the 1-level and the infinity level. If you look back at the last few diagrams, you will...
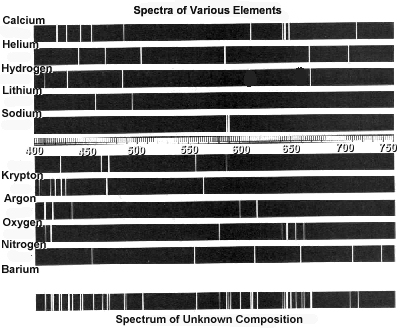
Practice Emission Spectra | PDF | Emission Spectrum | Energy Level Lab Methods: Emission Spectra and Energy Levels Practice. diffraction grating, a bright line spectrum, or line-emission spectrum is produced. Each element has its own unique emission spectrum by which it distinctive can be identified, analogous to a fingerprint.
Emission Spectra And Energy Levels Worksheet Emission spectra and energy levels worksheet answers can be valuable inspiration for those who seek a picture according specific classes yo...
Emission Spectrum of Hydrogen Emission Spectrum of Hydrogen. When an electric current is passed through a glass tube that To fit the observed spectrum, Planck had to assume that the energy of these oscillators could take on only a limited number of values. The energy levels of the hydrogen atom are quantized.
Hydrogen Spectrum - Emission, Absorption - Series, Diagram Atomic emission and absorption spectrum series, region and diagram for hydrogen atom, energy and frequency of spectra IR, UV, visible spectral lines. Hydrogen spectrum diagram. On the addition of thermal energy or electrical energy, the electron moved to the higher energy level or higher energy...
PDF Blackbody and Emission-Line Spectra Part 2 - Emission Spectra. Next, we will look at the spectra of different elements using the arc lamp, a simple device that passes an electric current through a A diagram of the hydrogen atom is shown at right: numbered circles designate the individual energy levels; their distance from the nucleus is...
PDF Microsoft Word - atomic_spectroscopy_2005.doc Note that the energy levels of the rotational states given by this expression are not equally spaced. 2/15/06. 10 Atomic and Molecular Spectroscopy. 1. Any OOIBASE spectrum saved as the scope output (with .sco extension) can be imported di-rectly into Origin worksheet.
PDF Emission spectra and energy levels worksheet A worksheet of emission spectrums and energy levels. Some of the worksheets shown are energy levels and atomic spectrum atom structure practices 1 working response lab report atomic emissions spectra emission spectra model practice work in response chapter 5 electrons atomic...
quantum mechanics - Why do atoms emit a certain colour of light? Atomic line emission and absorption spectra come from the differences between the discrete energy levels in the atom, and those are the solutions of a complex There are multiple energy levels in all atoms that electrons can occupy so there are many possible colours even for the simplest single atoms.

























0 Response to "40 emission spectra and energy levels worksheet"
Post a Comment