43 empirical and molecular formulas worksheet
Empirical And Molecular Formula Teaching Resources | TpT This empirical and molecular formula activity utilizes each student, encourages students to help each other, and offers a scaffolded approach to the in-class practice of empirical and molecular formulas through the rhyme: "percent to mass, mass to mole, divide by smallest, multiply 'til whole". Where applicable, answer key versions are set up usi Empirical and molecular formulas - The Cavalcade o' Chemistry Step 1: Find the molar mass of the empirical formula (i.e. treat the empirical formula as if it were a valid compound). In the case of CH₃, the molar mass would be 15.04 g/mol (12.01 for carbon, 3.03 for the three hydrogen atoms).
Empirical And Molecular Formula Worksheet - Balancing ... Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. 5 X 39 Y 13 X 2 Y. Empirical and Molecular Formulas INFORMATION An empirical formula is a lowest common denominator molecular formula for covalent molecules. Fill in the chart. In this worksheet we will practice using the structure of a compound to determine its ...
Empirical and molecular formulas worksheet
PDF Empirical Formula Worksheet - Roseville Joint Union High ... counting unit=the mole. It is important to remember that empirical formulas are experimental formulas and will always be the lowest common ratio between the elements. CHO is an empirical formula. C. 6. H. 12. O. 6. is NOT an empirical formula; all of the elements can be divided by 6! Empirical Formula: The simplest ratio of the atoms present in ... plaza.ufl.edu › ctoyota › worksheet 8Worksheet #8 Empirical Formulas H O N O 4I Answers to Worksheet #8 Empirical Formulas To calculate empirical formulas, follow the steps outlined below: (assume percentages given in the problems are grams) Step 1: convert to moles Step 2: divide each by the lowest number of moles Step 3: (only if necessary) multiply all by the same factor in order to obtain whole numbers. . chemistrymoleapplications.weebly.com › uploads › 7PERCENTAGE COMPOSITION WORKSHEET What is the empirical formula for a compound which contains 67.1% zinc and the rest is oxygen? (ZnO2) Barry Um has a sample of a compound which weighs 200 grams and contains only carbon, hydrogen, oxygen and nitrogen. By analysis, he finds that it contains 97.56 grams of carbon, 4.878 g of hydrogen, 52.03 grams of oxygen and 45.53 grams of ...
Empirical and molecular formulas worksheet. Empirical And Molecular Formula Worksheet Answers 6 10 Empirical And Molecular Formula Worksheet Answers 6 10 Author: autoadvisor.stevens.edu-2022-04-22T00:00:00+00:01 Subject: Empirical And Molecular Formula Worksheet Answers 6 10 Keywords: empirical, and, molecular, formula, worksheet, answers, 6, 10 Created Date: 4/22/2022 2:34:48 AM Empirical and Molecular Formula Worksheet Empirical and Molecular Formula Worksheet. SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C. 6H6 2) C8H18 3) WO. 2 4) C2H6O2 5) X39Y13 6) A compound with an empirical formula of C. 2. OH. 4. and a molar mass of 88 grams per mole. What is the molecular formula of this compound? Empirical And Molecular Formula Worksheet Answers 6 10 Empirical And Molecular Formula Worksheet Answers 6 10 Author: library.lnu.edu.ua-2022-04-22T00:00:00+00:01 Subject: Empirical And Molecular Formula Worksheet Answers 6 10 Keywords: empirical, and, molecular, formula, worksheet, answers, 6, 10 Created Date: 4/22/2022 8:46:46 AM Empirical And Molecular Formula Worksheet Answers 6 10 Molecular Formulas from Empirical Formulas. Problem Sets. Worksheet - Molar Mass, Answers; 28 of the above worksheet problems in an HTML file, Handwritten solutions; Some Bonus Empirical Formula Problems; Determine empirical formula from percent composition data 3.2 Determining Empirical and Molecular Formulas - Chemistry
Empirical and Molecular Formulas Empirical Formula = C. 4 . H. 5 . N. 2 . O. Ex1) Empirical Formula (cont.) 4.12 mol:4 1.03 mol. Carbon = 5.0 mol:5 1.03 mol. Hydrogen. ≈. 2.06 mol:2 1.03 mol. Nitrogen = 1.03 mol:1 1.03 mol. Oxygen = © 2010 High School Chem Solutions. All rights reserved. Worksheets - Empirical Formula - Arkansas State University Worksheets: Empirical Formula 1. Determine the empirical formula from the molecular formula: ... c) C 3 H 8: j) C 6 H 5 N: d) Fe 3 (CO) 9: k) P 4 O 10: e) C 2 H 4 O 2: l) Re 2 Cl 6: f) N 2 H 4: m) Se 3 O 9: g) CaBr 2: n) LiCl: 2. Determine the empirical formula from the percent composition for each of the following: ... A compound analyzes as ... Empirical And Molecular Formula Worksheets - Learny Kids Empirical And Molecular Formula. Displaying top 8 worksheets found for - Empirical And Molecular Formula. Some of the worksheets for this concept are , Empirical and molecular formula work, Empirical molecular formulas student notes, Work 8 empirical formulas h o n o 4i, Empirical formula work, Empirical formula work, Empirical and molecular ... DOC Molecular Formula Worksheet - Tamaqua Area School District 7. Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 32.06 g/mol. 1. Empirical Formula = P2O5 Empirical Mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 Molecular Mass = 283.89g. 2(P2O5) = P4O10 2. Empirical Formula = CH Empirical Mass = 1(12.0) + 1(1.0) = 13g
› definition-of-formula-mass-605144Formula Mass: Definition and Example Calculation Feb 05, 2020 · The molecular formula for glucose is C 6 H 12 O 6, so the empirical formula is CH 2 O. The formula mass of glucose is 12+2(1)+16 = 30 amu. Relative Formula Mass Definition PDF Empirical and Molecular Formula Worksheet Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C6H6 2) C8H18 3) WO2 4) C2H6O2 5) X39Y13 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. Empirical and Molecular Formula Worksheet Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER. Write the empirical formula for the following compounds. 1) C6H6 CH 2) C8H18 C4H9 3) WO2 WO2 4) C2H6O2 CH3O 5) X39Y13 X2Y 6) A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. What is the molecular formula of this compound? Empirical And Molecular Formula Worksheet Answers 6 1 Empirical And Molecular Formula Worksheet Answers 6 1 Author: net.as.gov-2022-04-19T00:00:00+00:01 Subject: Empirical And Molecular Formula Worksheet Answers 6 1 Keywords: empirical, and, molecular, formula, worksheet, answers, 6, 1 Created Date: 4/19/2022 3:10:32 PM
WRK- Empirical and Molecular Formula Worksheet - Empirical and Molecular Formula Worksheet SHOW ...
Molecular Formula Worksheet - worksheet Empirical and molecular formula worksheet. It starts with identifying molar mass from the periodic table and works up to percent composition breaking it down into steps and giving exampl. 6h6 2 c8h18 3 wo. Worksheets are empirical and molecular formula work empirical and molecular. Displaying all worksheets related to calculating molecular formula.
Molecular Formula Worksheet - Studying Worksheets April 18th 2019 Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 1 C6H6 C3H3 2 C8H18 C4H9 3 WO2 WO2 4 C2H6O2 CH3O 5 X39Y13 X3Y 6 A compound with an empirical formula of. The composition of the compound is 304 N and 696 O.
Grade 10,Empirical and molecular formula worksheet ID: 1610844 Language: English School subject: Chemistry Grade/level: 10 Age: 16-18 Main content: Stoichiometry Other contents: Add to my workbooks (6) Download file pdf Embed in my website or blog Add to Google Classroom
Empirical And Molecular Formula Worksheet Answers 6 10 2.6 Molecular and Ionic Compounds. 2.7 Chemical Nomenclature. Chapter 3. Composition of Substances and Solutions . Introduction. 3.1 Formula Mass and the Mole Concept. 3.2 Determining Empirical and Molecular … 25/08/2021 · So you need two iron ions, (3 * 2 = 6), Fe 2, and three chromate ions, (2 * 3 = 6), (CrO 4) 3, to make a neutral compound.
Empirical And Molecular Formulas Activity Teaching ... Empirical and Molecular Formula Activity Worksheet Doodle Notes by Big Picture Teaching 6 $2.00 PDF This activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps.
› test-prep › mcatStoichiometry questions (practice) - Khan Academy Empirical formula from mass composition edited. Molecular and empirical formulas. The mole and Avogadro's number. Stoichiometry example problem 1. Stoichiometry.
Empirical Formula And Molecular Formula Worksheet Apr 19, 2022 · Empirical and molecular formula worksheet show work on a separate sheet of paper. Use the same strategy as problem #6. 2) What Is Empirical Formula Of A Compound Which Consists Of 89.14% Au And 10.80% Of O? Empirical and molecular formula worksheet 1. Ex2) molecular formula ex2) the molar mass of caffeine is 194.2 g/mol. • be able to ...
Empirical And Molecular Formula Worksheets - Kiddy Math Some of the worksheets for this concept are , Empirical and molecular formula work, Empirical molecular formulas student notes, Work 8 empirical formulas h o n o 4i, Empirical formula work, Empirical formula work, Empirical and molecular formula work, Calculating empirical and molecular formulas. Found worksheet you are looking for?
Empirical And Molecular Formula Worksheet - Agaliprogram Empirical And Molecular Formula Worksheet An Oxide Of Chromium Is Found To Have The Following % Composition: Chemistry unit 5 worksheet 3. 2 4) c2h6o2 5) x39y13 6) a compound with an empirical formula of c. 3) wo 2 wo 2. The Percent Composition Of A Compound Was Found To Be 63.5 % Silver, 8.2 % Nitrogen, And 28.3 % Oxygen.
11. Empirical Formula Worksheet II EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.
Empirical And Molecular Formulas Answer Key Worksheets ... Some of the worksheets for this concept are , Empirical molecular formulas student notes, Empirical formula work answers, Work 8 empirical formulas h o n o 4i, Empirical formulas answer key, Empirical and molecular formula work, Formula work, Percent composition and molecular formula work. Found worksheet you are looking for?
Practice Work 28: Empirical and Molecular Formulas Determine the molecular formula. 8 min max DETERMINING MOLECULAR FORMULA Step 1: The molecular mass was given. (58.12 g/mol) Step 2: Determine the empirical formula DETERMINING EMPIRICAL FORMULA Step 1: Mass was given. Skip to Step 2. Step 3 Mass Values Step 4 Step 5 Step 6: Step 7: Step 8 Va ( ) 49.98 g C 1 (1 mol C 12.01 g C
Empirical_Formula_Practice_1.pdf.docx - Name: _ Period ... What is its empirical and molecular formula? Empirical Formula:_____ Molecular Formula:_____ 7) The percent composition of an unknown substance is 75.42 % Carbon, 6.63 % Hydrogen, 8.38 % Nitrogen, and 9.57 % Oxygen. If its molar mass is 334.0 g/mol what is its empirical and molecular formula?
Lesson Worksheet:Empirical and Molecular Formulas | Nagwa In this worksheet, we will practice defining, determining, and converting between a compound's empirical and molecular formulas. Q1: Caffeine has the chemical formula C H N O 8 1 0 4 2. What is its empirical formula? A C H N O 4 5 2. B C H N O 8 1 0 4 2. C C H N O. D C H N O 1 6 2 0 8 4.
Chapter 8 Empirical and Molecular Formulas Worksheet 1 key ... Empirical and Molecular Formulas Worksheet 1 1. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic acid. a. The molar mass for question #1 was determined by experiment to be 60.0 g/mol.






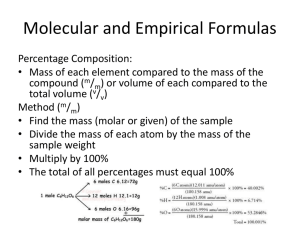



0 Response to "43 empirical and molecular formulas worksheet"
Post a Comment