40 worksheet 7 oxidation reduction reactions answers
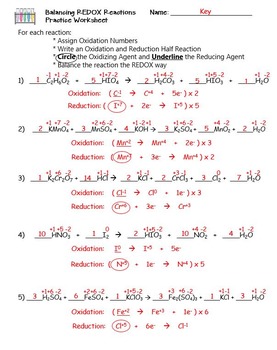
bkhakharia.files.wordpress.com › 2019 › 11Chem 12 Practice Worksheet - Answer Key Chem 12 Practice Worksheet - Answer Key Key page 1 Redox #1 (KEY) 1. Explain the meaning of each of the following terms: a) oxidation a half-reaction that involves the loss of electron(s) Chem 12 Practice Worksheet - Answer Key Chem 12 Practice Worksheet - Answer Key Key page 1 Redox #1 (KEY) 1. Explain the meaning of each of the following terms: a) oxidation a half-reaction that involves the loss of electron(s) b) reduction a half-reaction that involves the gain of electron(s) c) reducing agent a species that causes another to be reduced; it itself is oxidized d) oxidizing agent a species that causes …
Waves Review - Answers #1 - Physics Classroom The Physics Classroom serves students, teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional. Written by teachers for teachers and students, The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers.
Worksheet 7 oxidation reduction reactions answers
DOC Oxidation Reduction Worksheet - San Diego Mesa College For the following balanced redox reaction answer the following questions 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of oxygen in H2O2? -1 What is the element that is oxidized? Fe What is the element that is reduced? O What is the oxidizing agent? H2O2 What is the reducing agent? Fe+2 PDF Chapter 20 Worksheet Redox - Beverly Hills High School Chapter 20 Worksheet: Redox ANSWERS I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO Sr 0 to Sr2+; oxidized/reducing agent O0 to O2-; reduced/ox. ag. 2. 2Li + S Li2S Li 0 to Li1+; oxidized/red. ag. S0 to S2-; reduced/ox. ag. 3. PDF 2-5 Redox Reactions Practice Worksheet with Answers Which is an oxidation-reduction reaction? A. 4Na+O2!2Na2O B. 3O2!2O3 C. AgNO3+NaCl !AgCl+NaNO3 D. KI !K++I 21. Which is a redox reaction? A. CaCO3!CaO+CO2B. NaOH+HCl !NaCl+H2O C. 2NH4Cl+Ca(OH)2!2NH3+2H2O+CaCl2D. 2H2O !2H2+O2 page 2 Redox practice worksheet 22.
Worksheet 7 oxidation reduction reactions answers. Oxidation Number Exercise - Multidict oxidation-reduction reactions, 3) calculations in electrochemistry and other areas of chemistry. Rule 0 The following rules are in the form of a hierarchy; that is, the first stated rule takes precedence over subsequent rules if a conflict arises. Rule 1 The oxidation numbers for all the atoms in a neutral molecule must add up to 0. Similarly, the oxidation numbers for all the … PDF Redox Worksheet Key - humbleisd.net There are two kinds of reactions in the world, Oxidation-Reduction Reactions and Acid-Base Reactions. In a redox reaction, electrons are gained and electrons are lost. ... Which of the following reactions is (are) oxidation-reduction reactions? Explain your answer briefly. classify the remaining reactions. a) CdC12(aq) + Na2S(aq) Cds(s) + 2NaC1(aq) worksheet_25_oxidation_reduction_reactions_answers.pdf Worksheet 7 - Oxidation / Reduction of reaction Oxidation number of rules: Elements have oxidation number 0 Group I and II - In addition to elementary oxidation state 0, Group I has an oxidation state of +1, and group II oxidation state +2. accompanied by guides you could now enjoy is a worksheet of 7 responses to reducing oxidation below. 4.2 Classifying Chemical Reactions – Chemistry Oxidation-reduction (redox) reactions are those in which one or more elements involved undergo a change in oxidation number. (While the vast majority of redox reactions involve changes in oxidation number for two or more elements, a few interesting exceptions to this rule do exist Example 4.) Definitions for the complementary processes of this reaction class are …
PDF Worksheet 5.8 Answers - cpb-ap-se2.wpmucdn.com Chemical reactions: Summary Answers ... Worksheet 5.8 Science Quest 10: pages 226-7 reactants products created destroyed formulae aqueous precipitate nitrate potassium transfer oxidation reduction corrosion displacement hydrogen carbon decomposition reactivity hydrogen salt carbon dioxide catalyst enzyme PDF Worksheet 7.2: Solutions Rate of reaction No. Answer 10The reaction of hydrochloric acid with magnesium is a redox reaction: 2HCl(aq) + Mg(s) MgCl 2 (aq) + H 2 (g) The higher concentration of hydrochloric acid ensures that more molecules of HCl are available to react with the magnesium metal, and so the rate at which the reaction proceeds is greater. opentextbc.ca › 4-2-classifying-chemical-reactions4.2 Classifying Chemical Reactions – Chemistry Answers to Chemistry End of Chapter Exercises. 2. (a) oxidation-reduction (addition); (b) acid-base (neutralization); (c) oxidation-reduction (combustion) 4. It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction. 6. Oxidation-Reduction Worksheet - DocsLib Oxidation-Reduction Worksheet Oxidation-Reduction Worksheet For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, the reducing agent, the oxidation half reaction, the reduction half reaction. 1.Mg + HCl à MgCl2 + H2 2.Fe + V2O3 à Fe2O3 + VO 3.KMnO4 + KNO2 + H2SO4 à MnSO4 + H2O + KNO3 + K2SO4
study.com › cathode-anode-half-cell-reactionsCathode vs. Anode: Half Cell Reactions - Study.com Nov 02, 2021 · The anode is the site of the oxidation half-reaction, while the cathode is the site of the reduction half-reaction. Electrons flow away from the anode toward the cathode. Register to view this lesson DOC Oxidation-Reduction Worksheet - New York Science Teacher Oxidation Reduction Worksheet Answers 1. Mg0 + 2H+1 Cl-1 Mg+1 Cl2-1 + H20 -2e- 2(+1e-) 2. 0 +3 -2 3 -2 +2 -2 2Fe + 3V2O3 Fe2O3 + 6VO 2(-3e-) 3(+2e-) PDF 7 Oxidation, reduction, and AQA Chemistry redox reactions Answers to ... 7 Oxidation, reduction, and redox reactions Answers to practice questions Question number Answer Marks Guidance 1 (a) a reducing agent gives electrons 1 This must not refer to electron pairs. 1 (b) Zero 1 1 (c) (i) (+)3 1 You will always need to work out oxidation states so you need to 1 (c) (ii) -3 practise them a lot. PDF Worksheet 7 - Oxidation/Reduction Reactions 0 II +1 +2 -1 Worksheet 7 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
butane.chem.uiuc.edu › anicely › chem102Dfa10Worksheet 25 - Oxidation/Reduction Reactions 0 II +1 +2 -2 -1 Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1. ...
PDF Chapter 7 - An Introduction to Chemistry: Oxidation-Reduction Reactions 250 Chapter 7 Oxidation-Reduction Reactions 7.1 An Introduction to Oxidation-Reduction Reactions ObjeCtive 2 ObjeCtive 2 Zinc oxide is a white substance used as a pigment in rubber, sun‑blocking ointments, and paint. It is added to plastics to make them less likely to be damaged by ultraviolet radiation and is also used as a dietary supplement.
Worksheet 7 - Oxidation/Reduction Reactions Oxidation number ... - YUMPU worksheet 7 - oxidation / reduction reactionsoxidation number rules:elements have an oxidation number of 0group i and ii - in addition to the elemental oxidation state of 0,group i has an oxidation state of +1 and group ii has anoxidation state of +2.hydrogen -usually +1, except when bonded to group i or group ii,when it forms hydrides, -1.oxygen …
study.com › learn › lessonWhat is Oxidation? | Examples, Reactions and Process - Study.com Sep 14, 2021 · Oxidation is a chemical process. It is defined as a process that occurs when atoms or groups of atoms lose electrons. Another way to define oxidation is when a chemical species gains oxygen or ...
› Waves-Review-AnswersWaves Review - Answers - Physics Classroom Answer: E. A, B, and C can be quickly ruled out since it shows the amplitude of the reflected and incident pulse to be the same size. An incident pulse would give up some of its energy to the transmitted pulse at the boundary, thus making the amplitude of the reflected pulse less than that of the incident pulse.
PDF Oxidation and Reduction Workbook revised 1A - Laney College Oxidation, Reduction, Agents, & Reactions. WS 1 2. Oxidation Numbers Spontaneous Reactions WS 2 ... Worksheet #1 Writing half reactions 1. Define each: Remember "Oil Rig": Oxidation is loss ... If the answer is no, write a balanced equation for the reaction that would occur. 4. Can you keep 1 M HNO 3 in an Au container? If the answer is no ...
PDF More Practice Balancing Redox - Livingston Public Schools Balancing Redox Equations WorkSheet Oxidation Number Method for Balancing Redox Equations 1. 2. 3. 4. 5. 6. 7. Assign oxidation numbers to all elements and identify ...
PDF Worksheet 7 Answers - University of Illinois Urbana-Champaign in each of the following reactions, assign oxidation numbers to all of the elements and identify the oxidizing and reducing agents and the change in oxidation number. 4 fe + 3 02 —+2 fe203 change in oxidation number oxidizing agent 0 reducing agent o 43 cro -+ 20h-—2 cr04 -+ h20 ange in oxidation number oxidizing agent reducing agent h20 change …
multidict.net › cs › 6722Oxidation Number Exercise - Multidict Purpose: This exercise is designed to teach the student how to assign oxidation numbers. Oxidation numbers are very important and are used for 1) naming compounds, 2) balancing oxidation-reduction reactions, 3) calculations in electrochemistry and other areas of chemistry. Rule 0 The following rules are in the form of a hierarchy; that is, the ...
DOCX Electrochemistry Worksheet - 226407558471730947.weebly.com REVIEW Electrochemistry Worksheet. Balance the following oxidation-reduction reactions using the half-reaction method. HCOOH (aq) + MnO. 4 - (aq) CO 2 (g) + Mn2+Acidic solutionIdentify the oxidizing agent Identify the reducing agent. HXeO. 4 - (s) XeO 6. 4- + Xe (g)Basic solutionIdentify the oxidizing agent . identify the reducing agent
Worksheet 7 Oxidation Reduction Reactions Answers .pdf - sonar.ptotoday Chem 12 Practice Worksheet - Answer Key Key page 1 Redox #1 (KEY) 1. Explain the meaning of each of the following terms: a) oxidation a half- reaction that involves the loss of electron(s) b) reduction a half-reaction that involves the gain of electron(s) c) reducing agent a species that
PDF Worksheet 7 Oxidation Reduction Reactions Answers Oxidation-Reduction Worksheet Oxidation-Reduction Balancing Additional Practice Problems Acidic Solution 1. Ag + NO 3-→ Ag+ + NO 2. Zn + NO 3-→ Zn2+ + NH4 + 3. Cr 2O 7 2-+ C2H 4O → C 2H 4O 2 + Cr 3+ 4.
Reactions of metals with acids producing salts - RSC Education Your teacher will show you how to test the gas being produced in these reactions. Choose one of the metals that reacts rapidly with the acids, and in a clean test tube add a piece of this metal to a 2–3 cm depth of one of the acids. This time place a cork loosely in the top of the test tube so that any gas produced escapes slowly. Light a wood splint, remove the cork and immediately hold …









0 Response to "40 worksheet 7 oxidation reduction reactions answers"
Post a Comment