41 ph and poh worksheet
PDF Calculating the [H3O ], [OH ], pH and pOH in Aqueous Solutions: Worksheet O+], [OH-], pH and pOH in Aqueous Solutions: Worksheet 1. The concentration of hydroxide ions, OH-(aq), in a solution at 25°C is 0.150 mol/L. Determine the concentration of hydronium ions, H 3 O+(aq), in the solution. 2. A solution of lithium hydroxide, LiOH(aq), is made by placing 2.00 mol of the base into 1.50 L of solution. Ph And Poh Teaching Resources | Teachers Pay Teachers This worksheet set guides students through the following topics:What is the pH Scale? The pH range of acids and bases (along with common acids/bases).
ph and pOH worksheet key.doc - Course Hero View ph and pOH worksheet key.doc from AA 1pH and pOH Practice Sheet 1 1) What is the pH of a 0.00235 M HCl solution? 2.62 2) What is the pOH of a 0.00235 M HCl solution? 11.37 3) What is the pH of a

Ph and poh worksheet
DOC pH & pOH B. If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution? KEY. Chemistry: pH and pOH calculations. Part 1: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 ... PDF pH and pOH Calculations - Just Only pH and pOH Calculations 1) Determine the pH of a 0.00340 M HNO3 solution. 2) Determine the pOH of a 0.00340 M HNO3 solution. 3) Determine the pH of a 4.30 x 10-4 M NaOH solution. 4) If a solution is created by adding water to 2.30 x 10-4 moles of NaOH and 4.50 x 10-6 moles of HBr until the final volume is 1.00 L, what is the pH of this solution? pH and pOH Practice Questions - chem-textbook Calculate [H 3 O + ], [OH − ], pH, and pOH for pure water at 40 °C. The ionization constant for water ( Kw) is at 60 °C. Calculate [H 3 O + ], [OH − ], pH, and pOH for pure water at 60 °C. Solution. = [H 3 O +] = [OH − ]; ; pOH = pH = 6.5156. Calculate the pH and the pOH of each of the following solutions at 25 °C for which the ...
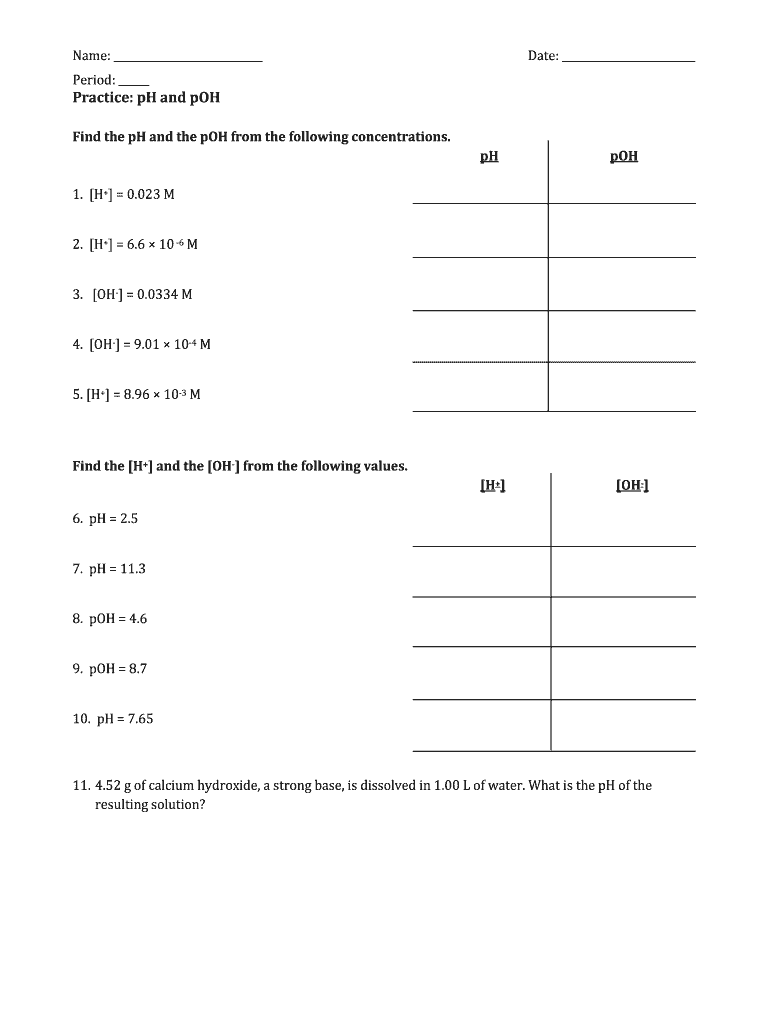
Ph and poh worksheet. PH and pOH worksheet - Liveworksheets.com ID: 1539333 Language: English School subject: Chemistry Grade/level: 12 Age: 16+ Main content: Acid-Base Equilibrium Other contents: pH and pOH Add to my workbooks (16) Download file pdf Embed in my website or blog Add to Google Classroom DOC pH and pOH Calculations Worksheet pH and pOH Calculations Worksheet #2 Find the pH and the pOH of the solution. Show your work and use correct sig. figs. pH pOH 1. A 0.023 M solution of hydrochloric acid 2. A 6.6 x 10 -6 M solution of nitric acid 3. A 0.0334 M solution of potassium hydroxide 4. A 1.0 x 10 -5 M solution of hydroarsenic acid 5. PDF Name: Date: pH and pOH Name: Date: pH and pOH About Chemistry Fill in the missing sections: [H+] pH [OH-] pOH Acid or Base 1. 1 x 10-6 2. 9 3. PDF Ph Worksheet Chemistry Answers Poh 2 36 11 64 poh 14 ph poh m14 2 36 log oh poh logoh 2 18 10 2 4 37 10 m 3 3 2nd log 2 36 oh oh 4 37 10 3 m 2 3 2 x 5 5710 3 mol ca oh l x mol ca oh 2 18m mol 2 2 3 2 2 0 412g ca oh 1 mol ca oh 74 g ca oh. Ph And Poh Worksheet Answers - worksheet Review: pH Calculations: Chemistry Quick Review of pH. 02. of 02. pH Worksheet Answers. Todd ...
PDF pH, pOH, Ka pKa worksheet - Mr. Bigler pH, pOH, K a & pK a worksheet Calculate the pH of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. 1. ... 7. pOH = 9.39 8. pH = 2.54. 9. An 0.100M solution of nitrous acid (HNO 2) has a pH of 2.17. (a) What is [H+] for this solution? Calculating pH and pOH - High School Chemistry - Varsity Tutors The question asks us to find the pH of the solution, so we will need to convert pOH to pH. To do so, we simply subtract the pOH from 14. The pH of the solution is 12.3. Because sodium hydroxide is a strong base, it makes sense that the pH is above 7. Report an Error Example Question #1 : Calculating P H And P Oh PDF pH pOH [H [OH Calculate the values of both pH and pOH of the following solutions: pH pOH a. 0.020 M HCl b. 0.0050 M NaOH c. A blood sample 7.2 x 10-8M of H+ d. 0.00035 M KOH ... pH WORKSHEET 6. Fill in the following table: ACID [H+] [OH-] pH pOH BASE NEUTRAL 1 X 10 -3 1 X 10 -6 9 12 NEUTRAL 9.5 4.7 -2.0 X 10 3 5.0 X 10-11 4.35 ... pH and pOH Practice Worksheet - ThoughtCo Nov 30, 2018 · He holds bachelor's degrees in both physics and mathematics. Learn about ourEditorial Process Updated on November 30, 2018 01 of 02 pH Worksheet Todd Helmenstine This downloadable PDF worksheet is for students to practice calculating pH and pOHvalues from concentration values of H+and OH-ions.
Calculating pH and pOH worksheet - Everett Community College pH = -log[H+] = -log(0.0235) = 1.629 2) What is the pOH of a 0.0235 M HCl solution? pH = -log[H+] = -log(0.0235) = 1.629 pOH = 14.000 –pH = 14.000 –1.629 = 12.371 3) -What is the pH of a 6.50 x 103M KOH solution? pOH = -log[OH-] = -log(6.50 x 10-3) = 2.187 pH = 14.000 –pOH = 14.000 –2.187 = 11.813 PDF Calculating pH and pOH worksheet - Coach Coker's Chemistry pH = -log[H+] = -log(0.0235) = 1.629 2) What is the pOH of a 0.0235 M HCl solution? pH = -log[H+] = -log(0.0235) = 1.629 pOH = 14.000 -pH = 14.000 -1.629 = 12.371 3) -What is the pH of a 6.50 x 103M KOH solution? pOH = -log[OH-] = -log(6.50 x 10-3) = 2.187 pH = 14.000 -pOH = 14.000 -2.187 = 11.813 PDF Name: Date: pH and pOH Name: Date: pH and pOH About Chemistry Fill in the missing sections: [H+] pH [OH-] pOH Acid or Base 1. 1 x 10-6 6 1 x 10-8 8 Acid 2. 1 x 10 ... Calculating pH and pOH worksheet-3.doc - Course Hero Solutions Note: The significant figures in the concentration of [H+] or [OH-] is equal to thenumber of decimal places in the pH or pOH and vice versa. 1) What is the pH of a 0.0235 M HCl solution?pH = -log [H+] = -log (0.0235) = 1.629 pH = 1.629
ph and pOH worksheet.doc - Google Docs ph and pOH worksheet.doc - Google Docs. Name: Date: Hr: pH and pOH Practice Sheet 1. 1) What is the pH of a 0.0235 M HCl solution? 2) What is the pOH of a 0.0235 M HCl solution? 3) What is the pH of a 6.50 x 10-3 M KOH solution? (Hint: this is a basic solution – concentration is of OH -) 4) What is the pH of a 6.2 x 10-5 M NaOH solution?
DOCX pH and pOH - DeKalb County School District Author: John Bergmann and Jeff Christopherson Created Date: 02/28/2017 03:15:00 Title: pH and pOH Subject: Chemistry Keywords: pH, pOH, acid, base Category
DOC pH & pOH - Central Bucks School District B. If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution? KEY. Chemistry: pH and pOH calculations. Part 1: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 ...
PDF Acid-Base Strength, K , pH, pOH and % Dissociation Worksheet , pH, pOH and % Dissociation - Worksheet Use the Table of Relative Strengths of Acids and Bases (Appendix B in MHR or appendix C9 in Nelson) to answer the following questions/problems:
PDF Acid And Base Worksheet Answer Key IntroductionKa Kb Kw pH pOH pKa pKb H+ OH-Calculations - Acids \u0026 Bases, Buffer Solutions , Chemistry Review Acid and Base | Page 1/10. File Type PDF Acid And Base Worksheet ... WORKSHEET 8 (NOTES) The pH of a WEAK acid solution Calculate the pH of a 0.500 M aqueous solution of formic acid, HCOOH [K a =1.77 x 10-4] Step 1 List the major species
PDF pH and pOH Part 2: For each of the problems below, assume 100% dissociation. 1. A. Write the equation for the dissociation of hydrochloric acid. B. Find the pH of a 0.00476 M hydrochloric acid solution. 2. A.
pH and pOH Practice Questions - chem-textbook Calculate [H 3 O + ], [OH − ], pH, and pOH for pure water at 40 °C. The ionization constant for water ( Kw) is at 60 °C. Calculate [H 3 O + ], [OH − ], pH, and pOH for pure water at 60 °C. Solution. = [H 3 O +] = [OH − ]; ; pOH = pH = 6.5156. Calculate the pH and the pOH of each of the following solutions at 25 °C for which the ...
PDF pH and pOH Calculations - Just Only pH and pOH Calculations 1) Determine the pH of a 0.00340 M HNO3 solution. 2) Determine the pOH of a 0.00340 M HNO3 solution. 3) Determine the pH of a 4.30 x 10-4 M NaOH solution. 4) If a solution is created by adding water to 2.30 x 10-4 moles of NaOH and 4.50 x 10-6 moles of HBr until the final volume is 1.00 L, what is the pH of this solution?
DOC pH & pOH B. If the pH is 11.64 and you have 2.55 L of solution, how many grams of calcium hydroxide are in the solution? KEY. Chemistry: pH and pOH calculations. Part 1: Fill in the missing information in the table below. pH [ H3O1+ ] pOH [ OH1- ] ACID or BASE? 3.78 1.66 x 10-4 M 10.22 6.03 x 10-11 M Acid 3.41 3.89 x 10-4 M 10.59 2.57 x 10-11 ...

:max_bytes(150000):strip_icc()/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)

:max_bytes(150000):strip_icc()/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)



0 Response to "41 ph and poh worksheet"
Post a Comment