42 percent composition and empirical formula worksheet
Quiz & Worksheet - How to Calculate Percent Composition and Determine ... Worksheet 1. Which of the following calculations is NOT part of determining the empirical formula? Percentage composition Mass composition Composition in moles Moles to mass 2. What is the percent... Worksheets - Empirical Formula - Arkansas State University Worksheets: Empirical Formula 1. Determine the empirical formula from the molecular formula: a) C 6 H 6: h) C 2 H 2: ... j) C 6 H 5 N: d) Fe 3 (CO) 9: k) P 4 O 10: e) C 2 H 4 O 2: l) Re 2 Cl 6: f) N 2 H 4: m) Se 3 O 9: g) CaBr 2: n) LiCl: 2. Determine the empirical formula from the percent composition for each of the following: a) 92.24 % C; 7 ...
DOC Percent Composition and Molecular Formula Worksheet Find the molecular formula for a compound with an empirical formula of CFBrO and a molar mass of 254.7 grams per mole. A 50.51 g sample of a compound made from phosphorus and chlorine is decomposed. Analysis of the products showed that 11.39 g of phosphorus atoms were produced. Determine the mass of chlorine in the sample

Percent composition and empirical formula worksheet
DOCX Percentage Composition and Empirical & Molecular Formula Chemistry: Percentage Composition and Empirical & Molecular Formula Solve the following problems. Show your work, and always include units where needed. 1. A compound is found to contain 36.5% Na, 25.4% S, and 38.1% O. Find its empirical formula. 2. Find the empirical formula of a compound that is 53.7% iron and 46.3% sulfur. 3. % Composition, Empirical And Molecular Formula Teaching Resources | TpT Percent Composition, Empirical & Molecular Formulas Task Cards by Chemistry Corner 7 $3.50 Zip Differentiate this resource to meet your needs with the included editable task cards! You will find these task cards to be a very versatile teaching tool. PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1. Calculate the percent of nitrogen in urea, NH 2 CONH 2 2. Calculate the percentage of water in zinc sulfate heptahydrate. 3. Calculate the percentage of oxygen in potassium chlorate. 4. Calculate the percentage of tin in potassium stannate trihydrate, K 2 SnO 3 •2H 2 O
Percent composition and empirical formula worksheet. PDF 7-21 % Composition and Empirical Formulas wkst Part 2: Empirical Formulas Work each of the following problems. SHOW ALL WORK. 1. A compound is found to contain 63.52 % iron and 36.48 % sulfur. Find its empirical formula. 2. In the laboratory, a sample is found to contain 1.05 grams of nickel and 0.29 grams oxygen. Determine the empirical formula. Empirical Formulas And Percent Composition Worksheets & Teaching ... Percent Composition, Empirical Formulas, & Molecular Formulas Worksheet by Chem Queen 3 $1.50 Zip Here's a great practice worksheet that includes 10 questions. DOC % Composition, Empirical Formula and Molecular Formula Worksheet - Weebly % Composition, Empirical Formula and Molecular Formula Worksheet A sample of an unknown compound with a mass of 0.847 g has the following composition: 50.51 % fluorine and 49.49 % iron. When this compound is decomposed into its elements, what mass of each element would be recovered? F = 0.4278g Fe = 0.4192g PDF Worksheet #6 - My Chemistry Class Worksheet Questions Show work for ANY math problem. Include ALL units. 1) Write the empirical formula for C 2 H 6 2) Write the empirical formula for CH 2 O 3) Write the empirical formula for CH 3 COOH 4) Write the empirical formula for H 2 O 5) Calculate % composition of each element in KNO 3 K = 38.67%, N = 13.86%, O= 47.48% Worksheet #6
Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Andrew Tourney: 10/31 production test - concurrent upload 2 ... Andrew Tourney: 10/31 production test - concurrent upload 2 ... ... test Percent Composition and Empirical Formula Worksheet.pdf 10. A compound has an empirical formula of C 2 H 3 O and a molar mass of 172 g/mol. 11. Ibuprofen, a common headache remedy, has an empirical formula of C 7 H 9 O and a molar mass of approximately 215 g/mol. 12. Nicotine is 74.1% carbon, 8.6% hydrogen, and 17.3% nitrogen by mass. PDF Unit 5 - Percent Composition, Empirical Formulas, and Reactions Unit 5 - Percent Composition, Empirical Formulas, and Reactions Unit Goals: As you work through this unit, you should be able to: 1. Calculate the percent composition of a substance from its chemical formula or experimental data. ... Unit 5 Worksheet Packet % Composition Activity HW 2 Writing and Balancing Chemical Equations 5-7 Reaction ...
Molecular Mass Calculations - ThoughtCo Mar 11, 2019 · Formula or Molar Mass Worksheet Answers (pdf) Note About Molecular Mass and Isotopes The molecular mass calculations made using the atomic masses on the periodic table apply for general calculations, but aren't accurate when known isotopes of atoms are present in a compound. Empirical and Molecular Formula Worksheet What is the empirical formula of this oxide? 13. A sample of indium chloride weighing 0.5000 g is found to contain 0.2404 g of chlorine. What is the empirical formula of the indium compound? 14. An unknown compound was found to have a percent composition as follows: 47.0 % potassium, 14.5 % carbon, and 38.5 % oxygen. What is its empirical formula? PDF 7-23 More practice % Composition and Empirical Formulas wkst Part 2: Empirical Formulas Work each of the following problems. SHOW ALL WORK. 1. Determine the empirical formula of a compound containing 63.50 % silver, 8.25 % nitrogen, and the remainder oxygen. 2. A compound is found to contain 63 % manganese, Mn, and 37 % oxygen. What is the compound's empirical formula? Determining Empirical Formulas Worksheet Answers - Blogger Worksheet 7-3 Name Percent Composition Empirical Formulas Period Glencoe Chemistry pp. Show work on a separate sheet of paper. The empirical formula for the compound having the formula H2C2O4 is A C2H2 B CO2H C COH D C2O4H2 E COH2 2. The percent composition of a compound was found to be 635 silver 82 nitrogen and 283 oxygen.
University of South Carolina on Instagram: “Do you know a ... Oct 13, 2020 · I’m a real and legit sugar momma and here for all babies progress that is why they call me sugarmomma progress I will bless my babies with $2000 as a first payment and $1000 as a weekly allowance every Thursday and each start today and get paid 💚
PDF Percent Composition, Empirical and Molecular Formulas masses or percent composition. -STEP 2: If you are given % composition, turn it into grams by assuming a 100.0 g sample. NOTE: If you are given mass, you do not need to do this step. -STEP 3: Convert the masses to the number of moles of each element. How to calculate an empirical formula •
How to Calculate Mass Percent Composition - ThoughtCo Nov 24, 2019 · Mass percent composition is also known percent by weight. It is abbreviated as w/w%. For a solution, mass percent equals the mass of an element in one mole of the compound divided by the molar mass of the compound, multiplied by 100%.
DOC Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet Solutions 1) What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? C3H3O 2) If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? C6H6O2
DOC PERCENTAGE COMPOSITION WORKSHEET - The Mole PERCENTAGE COMPOSITION WORKSHEET PERCENTAGE COMPOSITION WORKSHEET 1. Calculate the COMPLETE percentage composition (by mass) of the following: (a) iron (III) oxide (Fe = 70.0%, O = 30.0%) (b) barium phosphate (Ba = 68.4%, P = 10.3%, O= 21.3%) 2. What is the percentage of sodium (by mass) in sodium phosphate? (42.1%) 3.
PDF 16 - Mass Percent and Empirical Formulas - Sacramento State Part A: Mass Percent Composition An element's mass percent composition is constant for each compound no matter how much of the ... CHM 4, PAL - Mass percent and empirical formulas Student name: 4 6) Diethylene glycol (used in antifreeze blending) has the composition: 45.27% C, 9.50 % H, and 45.23% O by mass. Its molar mass is 106.12 g/mol.
Chemistry 702: Percentage Composition and Empirical Formulas | Georgia ... Chemistry 702: Percentage Composition and Empirical Formulas Instructions. Before viewing an episode, download and print the note-taking guides, worksheets, and lab data sheets for that episode, keeping the printed sheets in order by page number. ... More Practice with Percentage Composition and Empirical Formulas Worksheet (60.21 KB) Chemistry ...
PDF Percent Composition and Molecular Formula Worksheet Percent Composition and Molecular Formula Worksheet 1. What's the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what's the molecular formula? 3. What's the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon ...




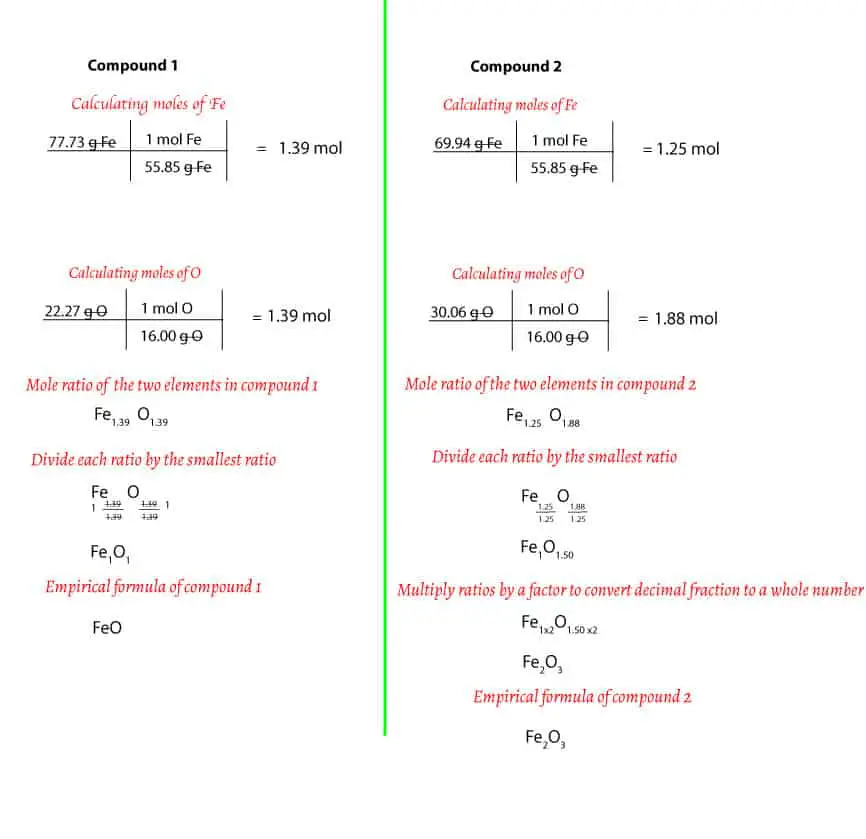
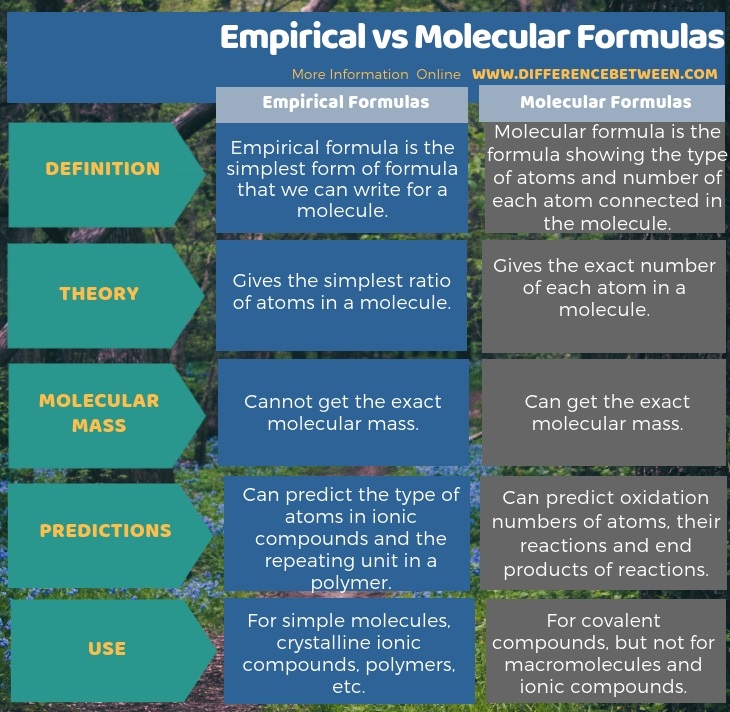
0 Response to "42 percent composition and empirical formula worksheet"
Post a Comment