43 more average atomic mass worksheet answers
Average Atomic Mass Worksheet.docx - Course Hero Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, silvery-white metal that has two common isotopes,85Rb and87Rb. If the abundance of85Rb is 72.2% and the abundance of87Rb is 27.8%, what is the average atomic mass of rubidium? 2) Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. PDF NAME Average Atomic Mass Worksheet: show all work. - Weebly and 24.47 percent 37Cl (mass = 36.966 amu). Calculate the average atomic mass. 35.46 amu 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63 ...
Quiz & Worksheet - Atomic Number and Mass Number | Study.com You will receive your score and answers at the end. question 1 of 3 ALL atoms of the same element have: different numbers of neutrons. the same number of neutrons. the same number of protons....
More average atomic mass worksheet answers
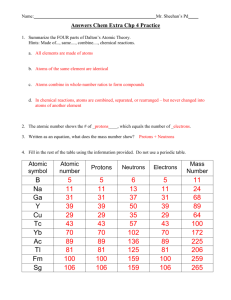
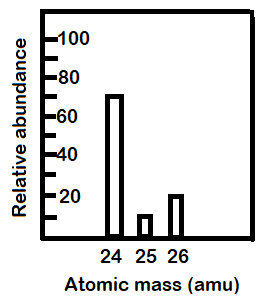
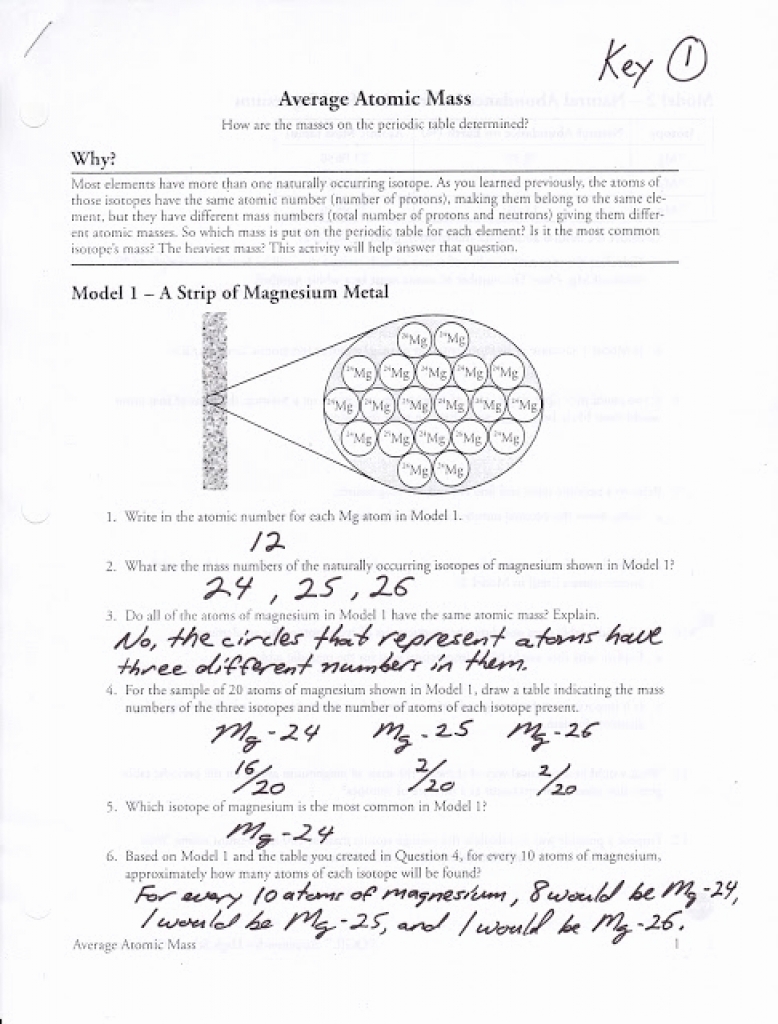
PDF Average Atomic Mass - Mr. Sault's Classroom a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11. Practice - Average Atomic Mass Worksheet 2.0 - Teachers Pay Teachers 4 Questions - 1 to calculate average atomic mass, 2 to calculate the atomic mass of an isotope, 1 to calculate the 2 natural abundances of an element with 2 isotopes like this --> Video. Answer Key sold separately " should be posted in a link here " Get both the worksheet and the answer key in the bundle above ^^ for 10% off! .docx file Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu.
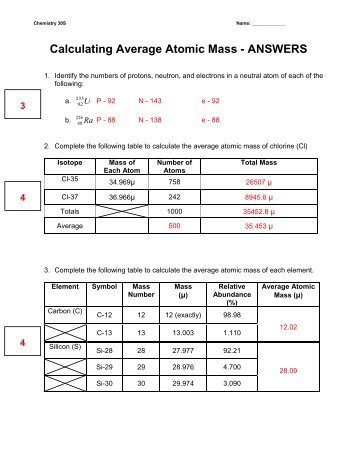
More average atomic mass worksheet answers. 2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. PDF Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key ... Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 ... DOC Chemistry Worksheet - MS. SMITH'S CLASS More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. PDF isotopic abundance practice problems - CHEMISTRY Using the average mass from the periodic table, calculate the abundance of each isotope.! atomic mass = mass 1 1)+ mass 2 2)+ . . . carbon 6 C 12.011 isotope % abundance mass (amu) carbon-12 99.45 12.000 carbon-14 0.55 14.003 atomic mass = (12.000 × 0.9945)+ (14.003 × 0.0055) atomic mass = (11.934)+ (0.077)= 12.011 amu
Average Atomic Mass Gizmo Answer Key ~ 22 Relative Mass And ... - Blogger Then, calculate the average atomic mass by considering the mass and abundance of each isotope. More specifically, ending at 12,011. How many electrons are in 23na? The atomic mass for each element appearing on the periodic table. Похожие запросы для average atomic mass gizmo answer key. ... Average atomic mass worksheet answer ... PDF More Average Atomic Mass - S.W.H.S CHEMISTRY More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. PDF Practice Problems - Denton ISD 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ... How to Find Average Atomic Mass: 8 Steps (with Pictures ... - wikiHow A molecule of water has the chemical formula H 2 O, so it contains two hydrogen (H) atoms and one oxygen (O) atom. Hydrogen has an average atomic mass of 1.00794 amu. Oxygen atoms have an average mass of 15.9994 amu. The average mass of a molecule of H 2 O equals (1.00794) (2) + 15.9994 = 18.01528 amu, equivalent to 18.01528 g/mol.
Quiz & Worksheet - Average Atomic Mass | Study.com Atomic mass Atomic number Number of electrons Number of protons Next Worksheet Print Worksheet 1. Which of the following indicates the percentage of the natural occurrence of an isotope of an... Practice - Average Atomic Mass Worksheet 1.1 - Answer Key Bundle - Average Atomic Mass Practice Worksheet 1.1 + Answer Key Save 5% off regularly priced items with this bundle!!The Chemistry Teacher WebsiteThe Chemistry Teacher on YouTube 2 Products $ 2.35 $ 2.48 Save $ 0.13 View Bundle Bundle - Average Atomic Mass Practice Worksheets 1.0, 1.1, & 2.0 + Answer Keys DOC Chemistry Worksheet - Humble Independent School District More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. Average Atomic Mass Worksheet.pdf - Name: _ Date: _ Period:... Average atomic mass = (mass x % abundance) + (mass x % abundance) + …… 100 1) Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is27.8%, what is the average atomic mass of rubidium? 2) Uranium has three common isotopes.
PDF More Average Atomic Mass - S.W.H.S CHEMISTRY More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2.
PDF Mass Spectrometer Answer Key - Livingston Public Schools The largest mass will represent the "average mass" of the molecule. When the molecule is passed through the ionization chamber, parts of it break off, or fragmentation occurs. If you were to remove the end carbon and the 3 hydrogen's bonded to it, the mass of the resulting ion (CH3CH2CH2CH2 ) would be 57. If you were remove the two end carbons ...
PDF Isotope Practice Worksheet - Chemistry Based on the atomic mass, which isotope should be more abundant? Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9.
PDF Answers Key for Unit Worksheets - Livingston Public Schools NAME Average Atorhic Mass Worksheet: work. 1) Rubidium is a soft, silvery-white metal that has two cornmon.isotopes 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, whatis the avétagè atomic mass of rubidium? amt) 2) Uranium is used in nuclear reactors and is a rare element on earth.
PDF AP Chemistry Day 12 - Ms. Fleming the worksheet. - Discuss with your group if needed - Even if you think you received full points, think about how you could rewrite your answer to express your ideas clearly, concisely, and in a logical flow. ... § We are more concerned with the average atomic mass.
Atomic Number And Atomic Mass Worksheets - K12 Workbook Displaying all worksheets related to - Atomic Number And Atomic Mass. Worksheets are Chemistry work atomic number and mass number, Atomic mass and atomic number work, Atomic structure work, Teacher workbooks, Example exercise atomic mass and avogadros number, Protons neutrons and electrons practice work, Preview, Basic atomic structure work key 1.
PDF Atomic Structure Worksheet Answers - Columbia Public Schools How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table.
Average Atomic Mass Practice Problems Quiz - Quizizz 6 Questions Show answers Question 1 900 seconds Q. 24.1% of all the isotopes of a an element have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average mass of this element? answer choices 74.92 amu 24.97 amu 75.01 amu 74.51 amu Question 2 120 seconds Q. Calcium has three different isotopes.
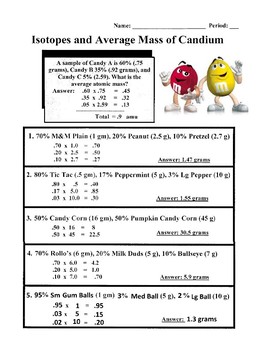
PDF KMBT 654-20131024112244 - Berger's Chemistry Class What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes. l. IZC 2. 500/0 3. 150/0 4. Il-I, 0.8%4-1, 0.2% 0.01b 5. OMS 13, B 0t46 6. 980/0 Chemistry IF87ó6
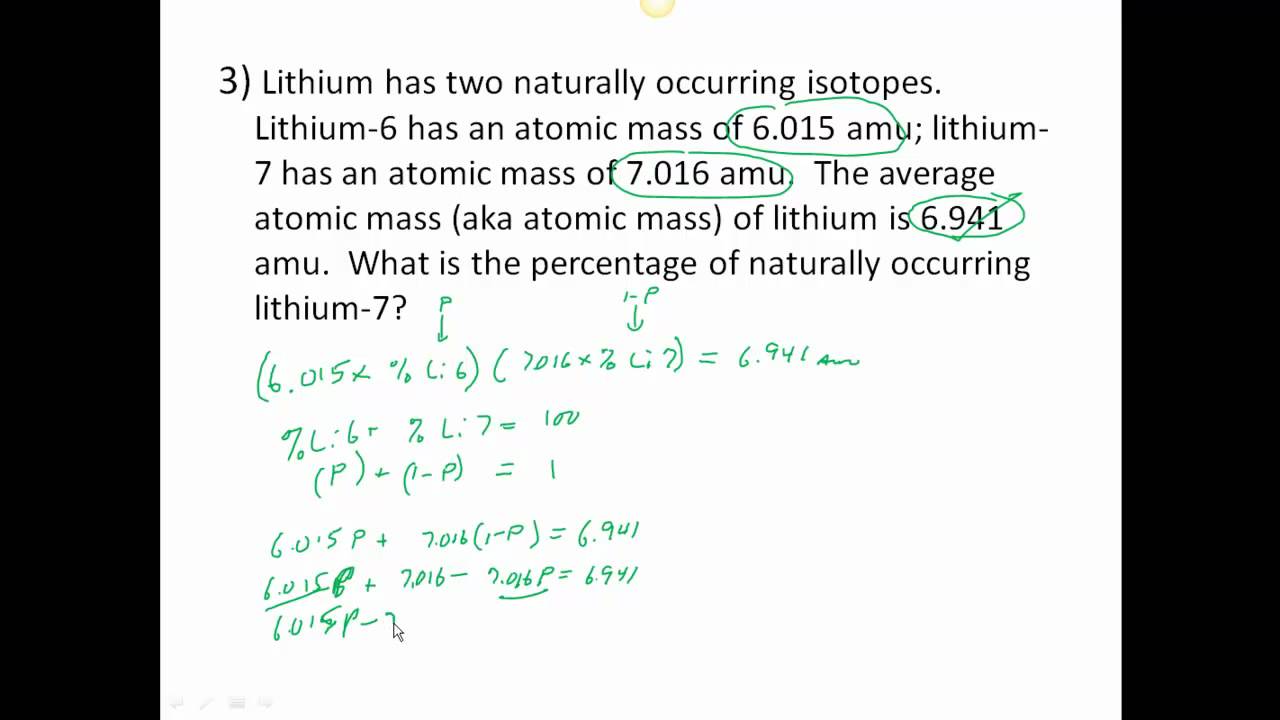
PDF CHM 4, PAL - atomic mass Student name - California State University ... CHM 4, PAL - atomic mass Student name: 2 Part B: Atomic mass calculations 4) Naturally occurring lithium consists of two isotopes, Li-6 with a mass of 6.015 amu and an abundance of 7.42% and Li-7 with a mass of 7.016 amu. Start by filling out the table below and then calculate the atomic mass of lithium. Show all your work
Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Find the average atomic mass for Cl is 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu.
Practice - Average Atomic Mass Worksheet 2.0 - Teachers Pay Teachers 4 Questions - 1 to calculate average atomic mass, 2 to calculate the atomic mass of an isotope, 1 to calculate the 2 natural abundances of an element with 2 isotopes like this --> Video. Answer Key sold separately " should be posted in a link here " Get both the worksheet and the answer key in the bundle above ^^ for 10% off! .docx file
PDF Average Atomic Mass - Mr. Sault's Classroom a. Which isotope has an atomic mass closest to the average atomic mass listed on the periodic table? 24.mg b. Give a mathematical reason for your answer to part a. 24 Mg is the most common isotope and is thus most heavily "weighted" in the equation for average atomic mass. 17. Boron has two naturally occurring isotopes: boron-10 and boron-11.






0 Response to "43 more average atomic mass worksheet answers"
Post a Comment