38 worksheet electrons in atoms
Covalent Bonds vs Ionic Bonds - Difference and Comparison | Diffen For example, let us consider a Methane molecule i.e.CH 4. Carbon has 6 electrons and its electronic configuration is 1s22s22p2, i.e. it has 4 electrons in its outer orbit. According to the Octate rule ( It states that atoms tend to gain, lose, or share electrons so that each atom has full outermost energy level which is typically 8 electrons.), to be in a stable state, it needs 4 more … The Science Spot The members of the Atoms Family correspond to protons, neutrons, and electrons to help students remember their charges and locations in an atom. In addition, students learn the basics of electron configuration with a tour of Matterville.
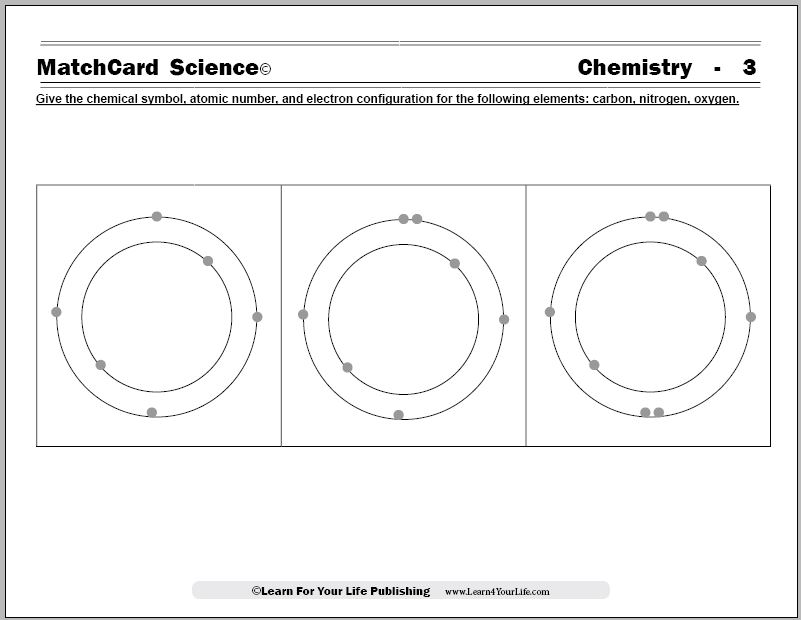
Chemistry of Matter - Science Spot 12. How many electrons can each level hold? 1st = 2 2nd = 8 3rd = 18 13. What term is used for the electrons in the outermost shell or energy level? VALENCE 14. Scientists use two types of diagrams to show the electron configuration for atoms. Follow your teacher’s directions to complete the diagrams. Sulfur Atomic # = 16 Atomic Mass = 32 ...
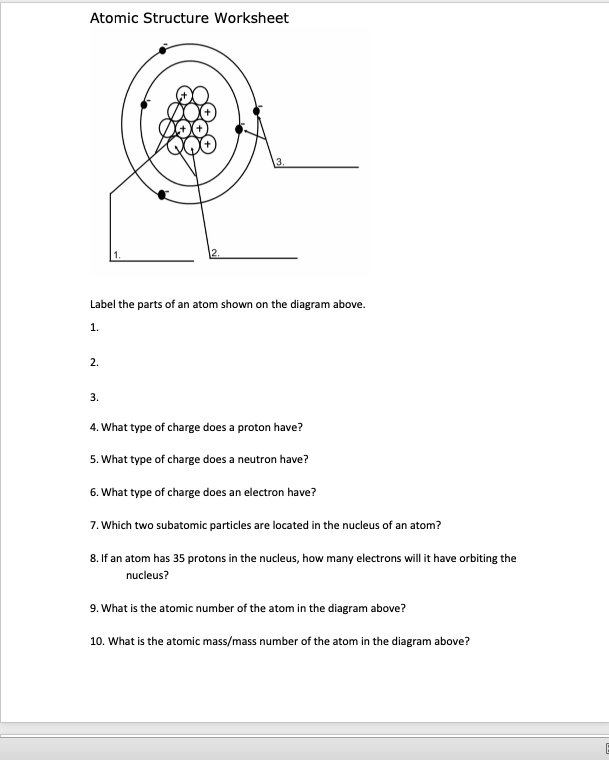
Worksheet electrons in atoms
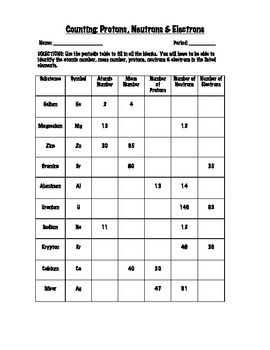
Basic Atomic Structure Worksheet Key - Neshaminy School District Give the symbol of and the number of electrons in a neutral atom of: Uranium Boron Chlorine Iodine Xenon Give the symbol of and the number of neutrons in one atom of: (Mass numbers are ALWAYS whole numbers...show your calculations) Barium Carbon Fluorine Europium IS-I 10 Bismuth Hydrogen Magnesium Mercury Isotope Worksheet Answer Key - ISD 622 # of electrons mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of Atomic Neutrons Electrons Atomic Charge Protons mass Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 +1 46 106 0 76 114 72 19 39 0 Mo 36 96 106 106 265 87 223 0 Hg 78 54 131 0 . Protons, Neutrons, and Electrons …
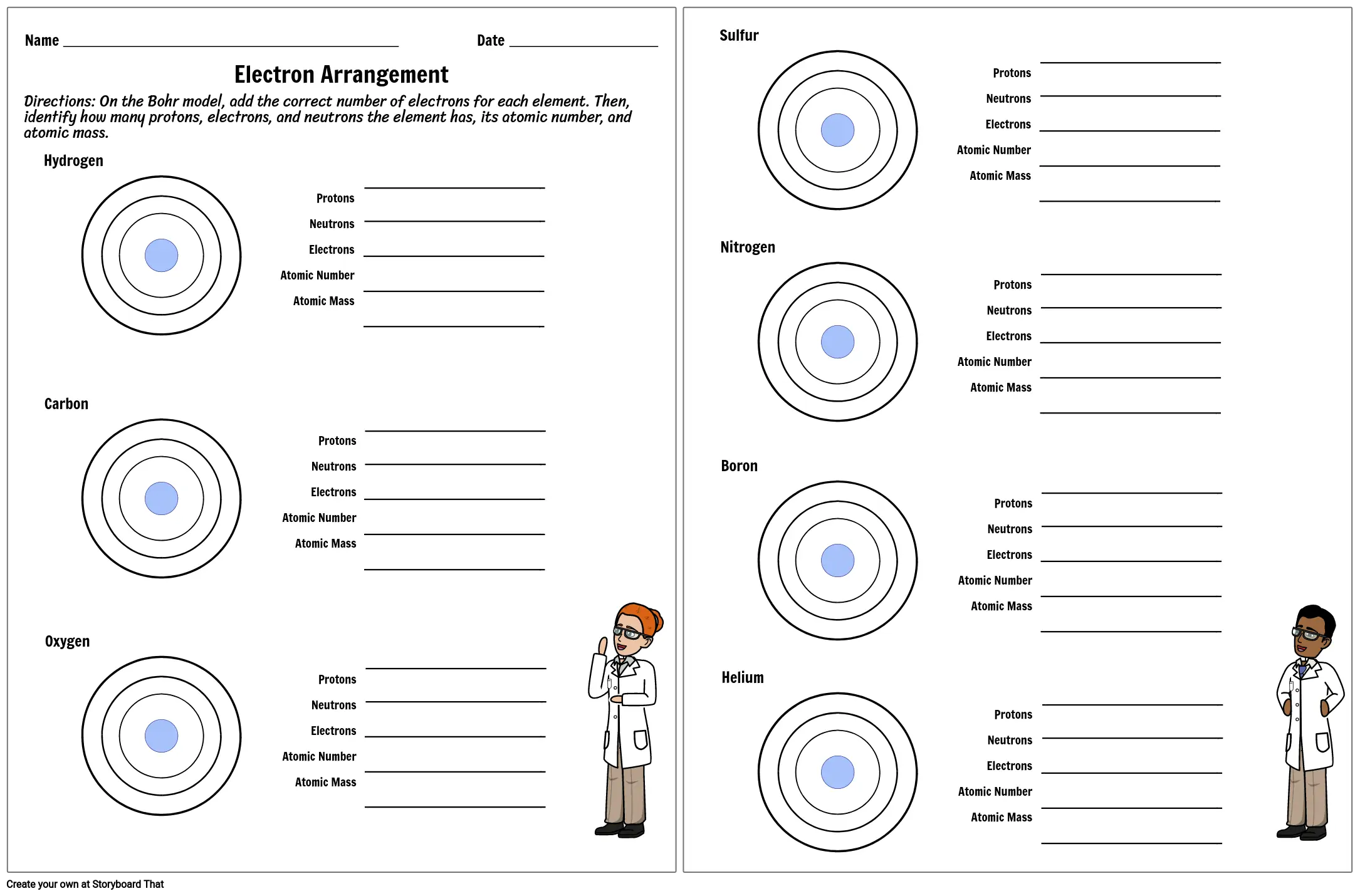
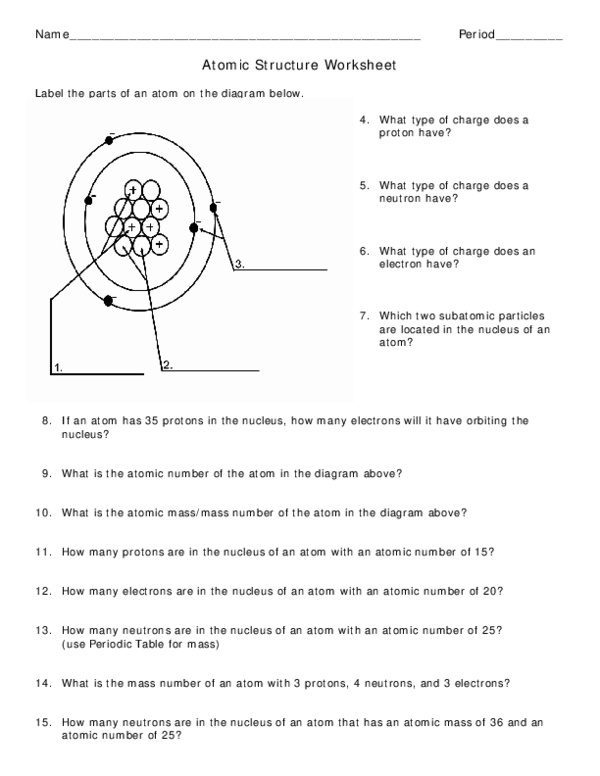
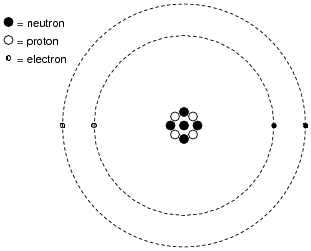
Worksheet electrons in atoms. Loading... - BrainPop Loading... - BrainPop ... Loading... Atomic Structure Worksheet - WILLAMETTE LEADERSHIP ACADEMY What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? 15. How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an Build an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Shapes of Molecules - Angelo State University When two bonded atoms have a difference of greater than 2.0 electronegativity units (see Table 2), the bond is an ionic bond — one atoms takes the electrons away from the other atom, producing cations and anions. For example Na has an electronegativity of 0.93, and Cl is 3.16, a difference of 2.23 units.
Chemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an … Oxygen - Element information, properties and uses | Periodic ... Today one gram can be beaten into a square meter sheet just 230 atoms thick, one cubic centimetre would make a sheet 18 square meters, 1 gram could be drawn out to make 165 meters of wire just 1/200 th of a millimetre thick. The gold colour in Buckingham Palace fence is actually gold; gold covered because it lasts 30 years; whereas gold paint ... electron | Definition, Mass, & Facts | Britannica 22. Sept. 2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and … Atom Worksheets Atom is the most basic unit of matter. It has a dense nucleus with a cloud of negatively charged electrons surrounding it. Here are the parts of an Atom: Electron; it is a subatomic particle with a negative electrical charge. The mass of an electron is so small that it is generally not even considered.
Atomic Neutrons Electrons Atomic Charge Protons mass Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 +1 46 106 0 76 114 72 19 39 0 Mo 36 96 106 106 265 87 223 0 Hg 78 54 131 0 . Protons, Neutrons, and Electrons … Isotope Worksheet Answer Key - ISD 622 # of electrons mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of Basic Atomic Structure Worksheet Key - Neshaminy School District Give the symbol of and the number of electrons in a neutral atom of: Uranium Boron Chlorine Iodine Xenon Give the symbol of and the number of neutrons in one atom of: (Mass numbers are ALWAYS whole numbers...show your calculations) Barium Carbon Fluorine Europium IS-I 10 Bismuth Hydrogen Magnesium Mercury



























0 Response to "38 worksheet electrons in atoms"
Post a Comment