42 chemistry atomic number and mass number worksheet answers
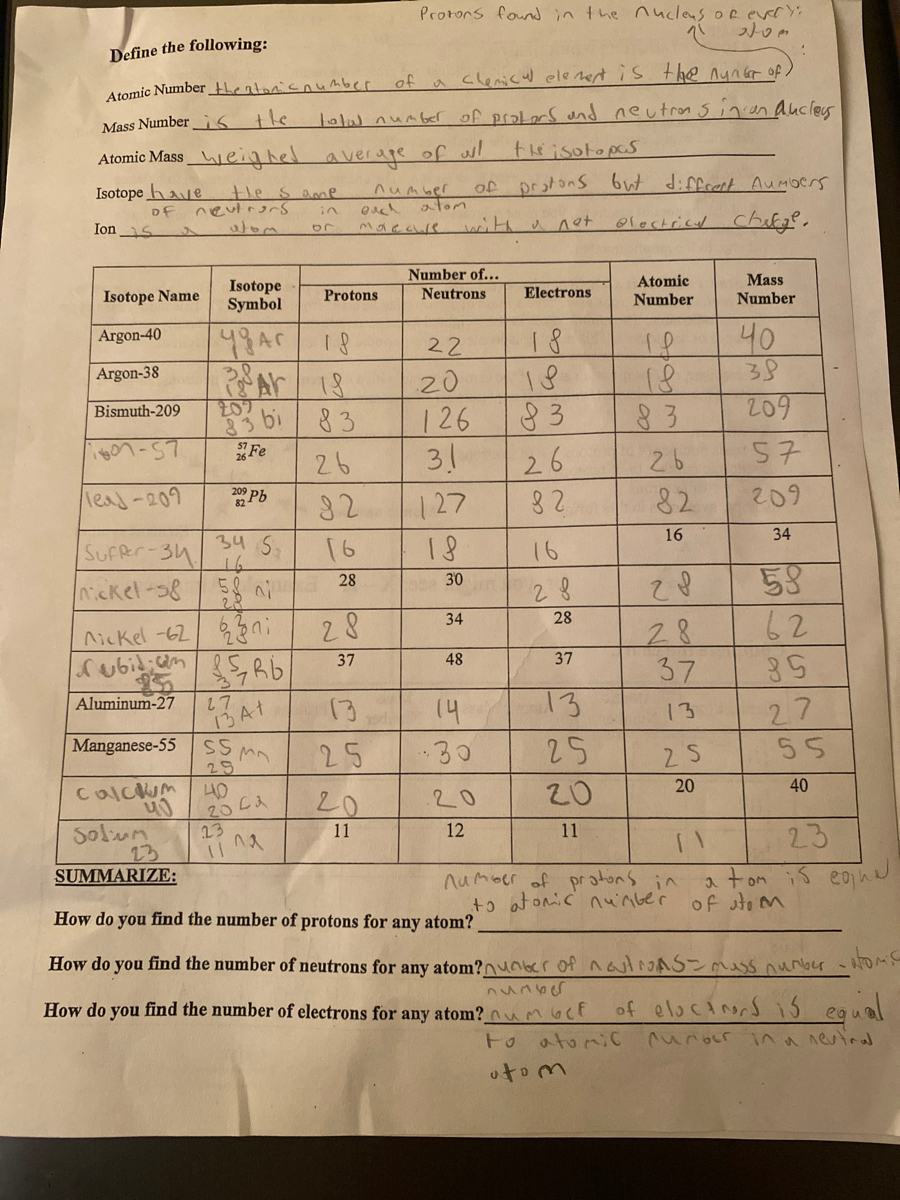
› molecular-mass-calculationsMolecular Mass Calculations - ThoughtCo Mar 11, 2019 · Find the atomic mass for each element by using the mass given in the Periodic Table. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. For example, multiple the subscript 12 times the atomic mass of carbon (C). Isotope Worksheet Answer Key - ISD 622 mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of
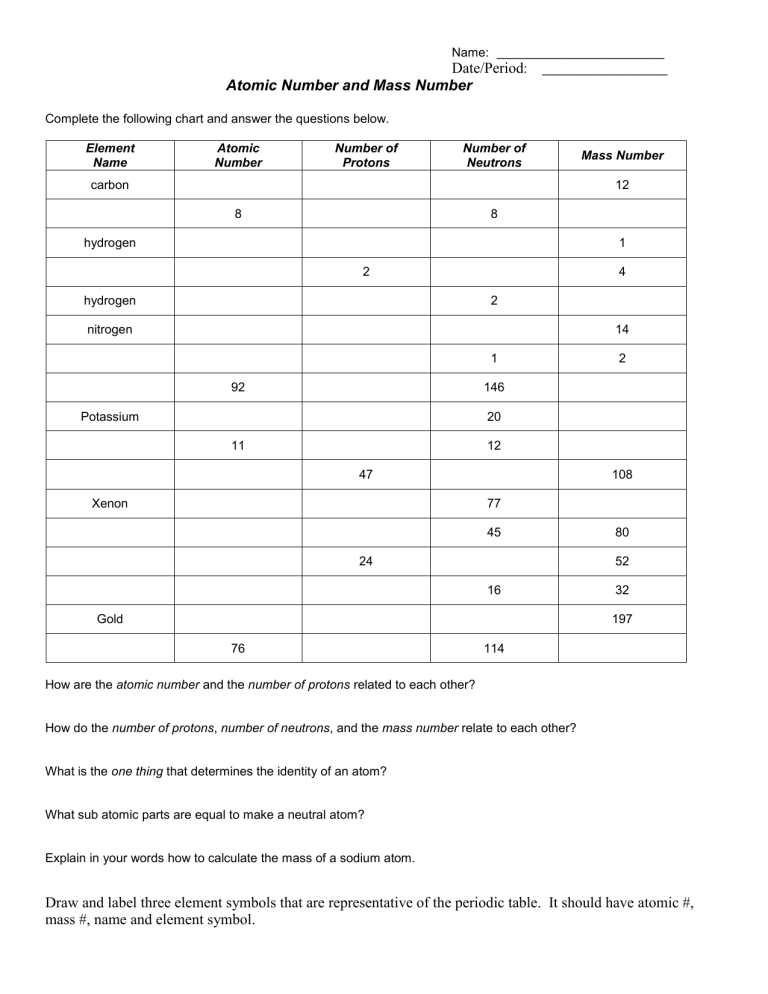
study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element?

Chemistry atomic number and mass number worksheet answers
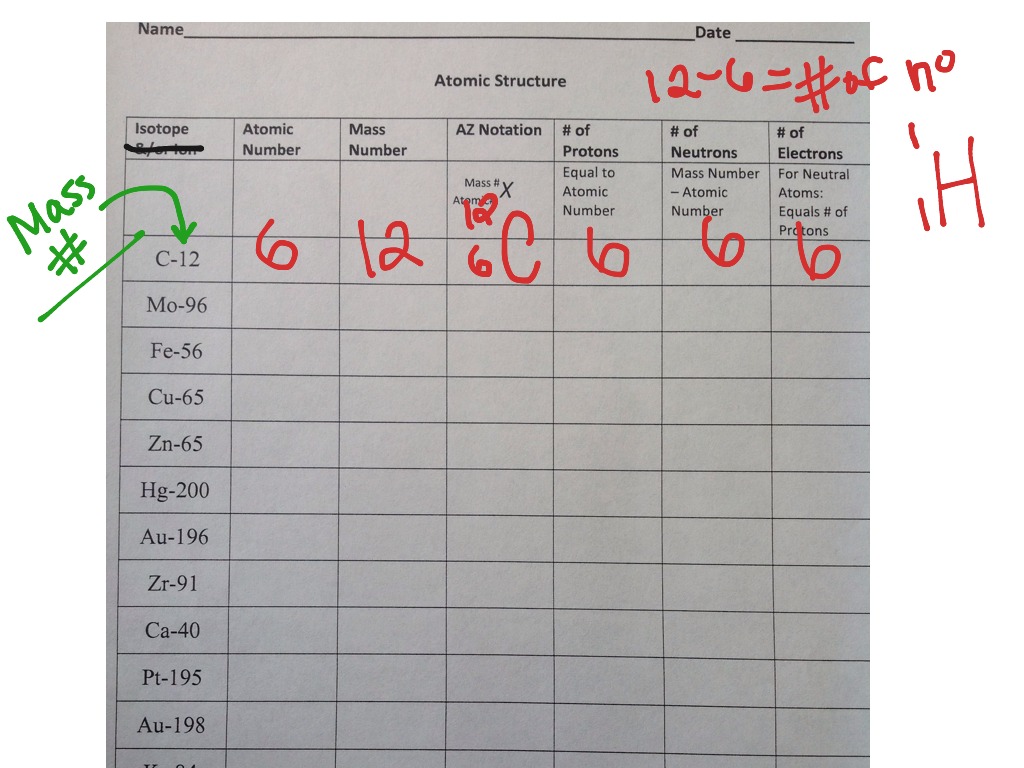
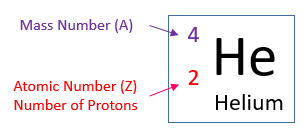
Atomic Structure Worksheet Answers - Columbia Public Schools Atomic Structure Worksheet Answers - Columbia Public Schools › cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.
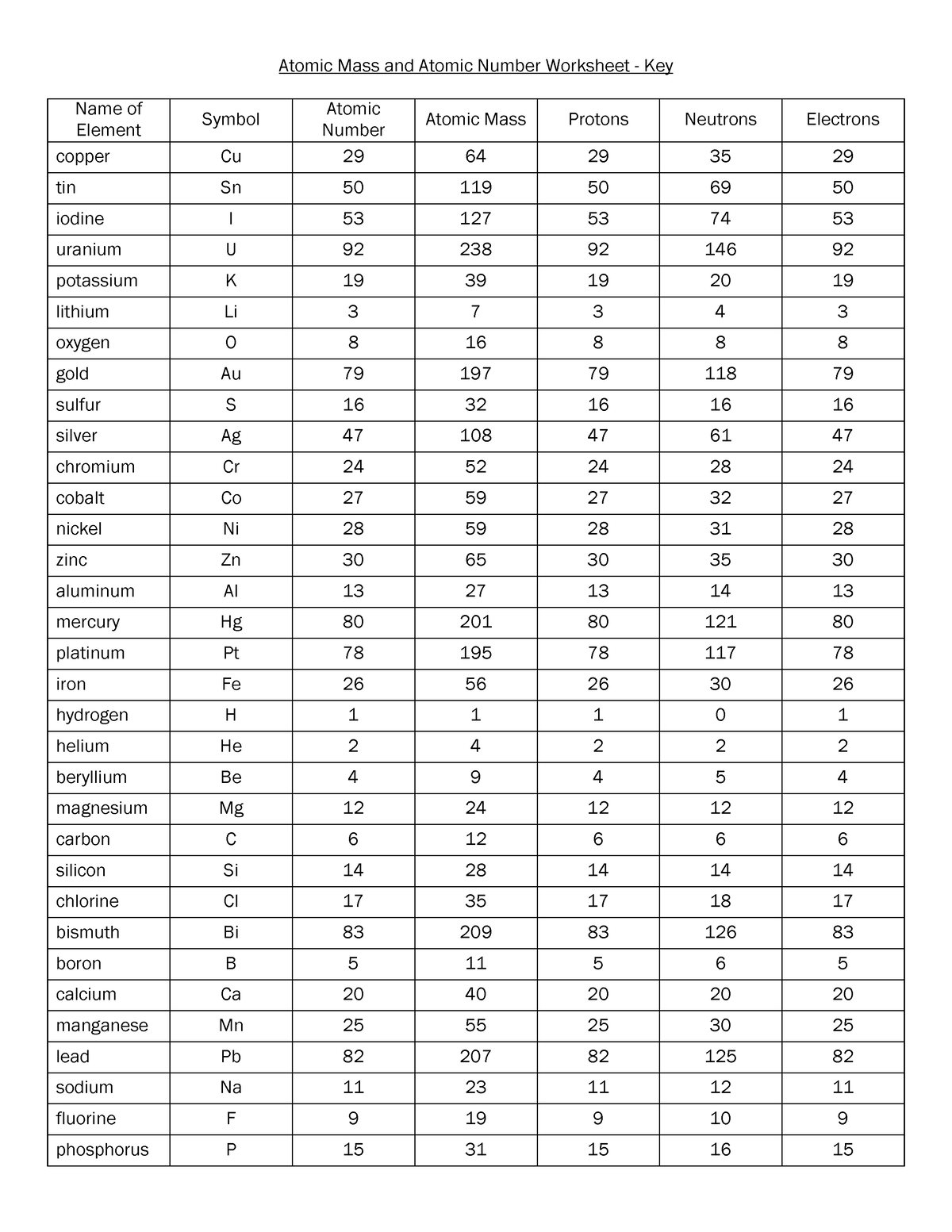
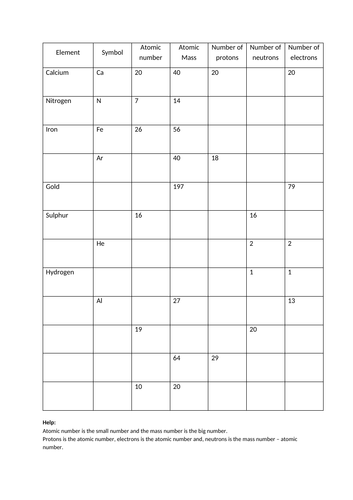
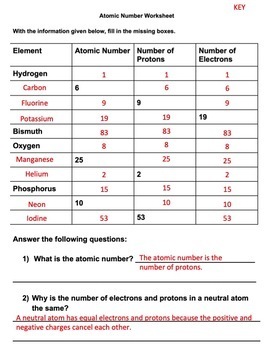
Chemistry atomic number and mass number worksheet answers. edu.rsc.org › resourcesTeaching resources | RSC Education Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities › publication › ppic-statewide-surveyPPIC Statewide Survey: Californians and Their Government Oct 27, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and state legislators and to make ... › cms › lib07Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In
en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. › cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In Atomic Structure Worksheet Answers - Columbia Public Schools Atomic Structure Worksheet Answers - Columbia Public Schools






















0 Response to "42 chemistry atomic number and mass number worksheet answers"
Post a Comment