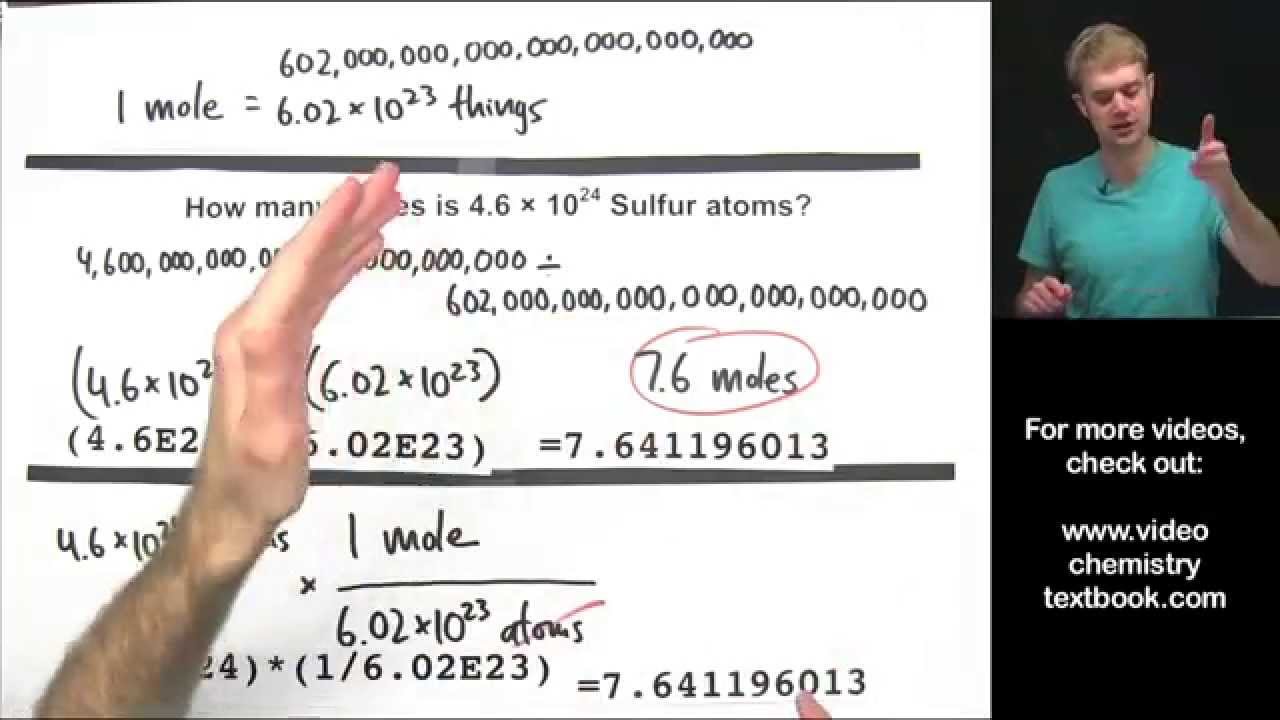
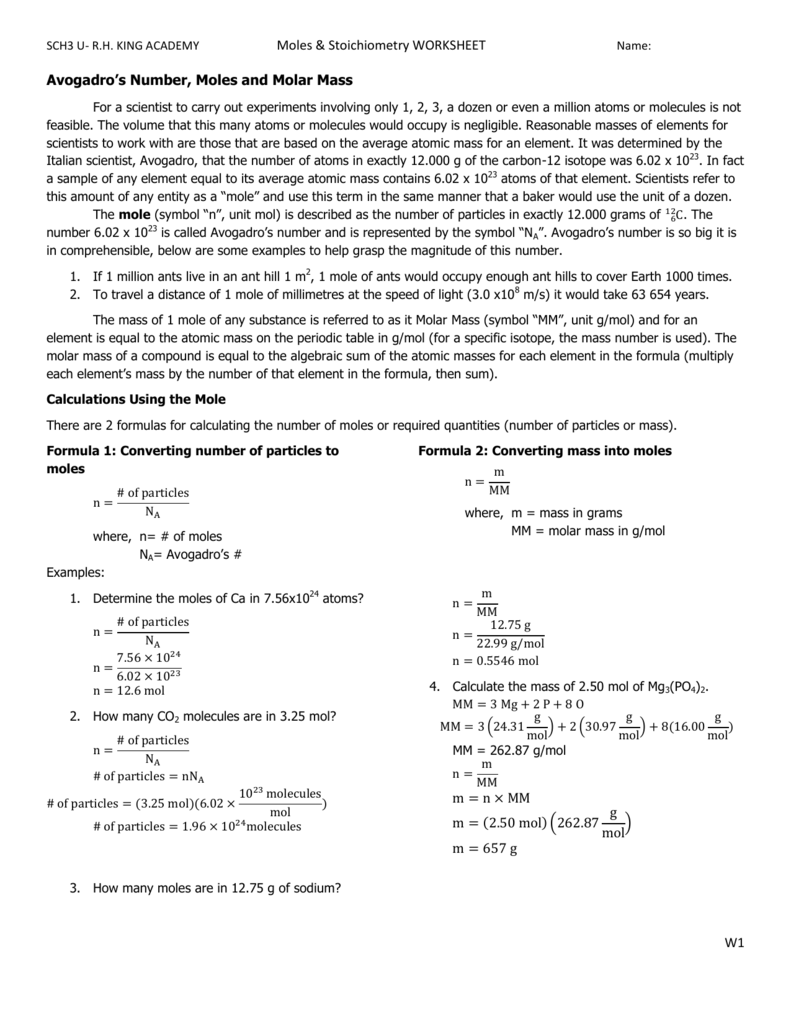
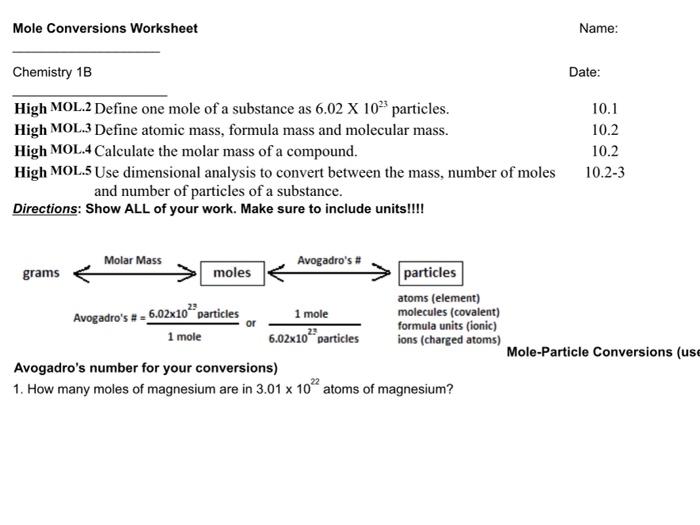
43 the mole and avogadro's number worksheet
Chemistry Study Guide for Gases - ThoughtCo Jul 03, 2019 · The mole fraction, X i, is calculated by dividing the number of moles of the individual gas by the total number of moles of the mixed gas. Avogadro's Gas Law Avogadro's law states the volume of a gas is directly proportional to the number of moles of gas when pressure and temperature remain constant. How to Calculate Mass Percent Composition - ThoughtCo Nov 24, 2019 · Step 2: Find the number of grams of each component make up one mole of CO 2. One mole of CO 2 contains 1 mole of carbon atoms and 2 moles of oxygen atoms . 12.01 g (1 mol) of C
lwtech-learning-lab-science-molar-mass mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole.

The mole and avogadro's number worksheet
Fundamentals of Analytical Chemistry- 9th Edition - Academia.edu Polyfunctional acids and bases play important roles in many chemical and biological systems. The human body contains a complicated system of buffers within cells and within bodily fluids, such as human blood. Gas Laws: Boyle's Law, Charle's Law, Gay-Lussac's Law ... Here, n is the number of moles of the gas. Hence, V= k 4 n. The number of molecules in a mole of any gas is known as the Avogadro’s constant and is calculated to be 6.022 * 10 23. The values for temperature and pressure here are the standard values. For temperature, we take it to be 273.15 K while for the pressure it equals 1 bar or 10 5 ... Solution - Definition, Properties, Types, Videos & Examples ... Solution - A solution is a mixture formed when a solid, liquid or gaseous substance is homogeneously mixed with a liquid. Likewise, a solvent is a substance in which another substance dissolves. To learn more about Properties, Types, Videos & Examples of Solution Visit BYJU'S.
The mole and avogadro's number worksheet. A Mole of Moles - xkcd It’s also, by chance, a decent ballpark guess for the number of grains of sand on Earth. A mole is also a type of burrowing mammal. There are a handful of types of moles, and some of them are truly horrifying. So what would a mole of moles—602,214,129,000,000,000,000,000 animals—look like? First, let’s start with wild ballpark ... Solution - Definition, Properties, Types, Videos & Examples ... Solution - A solution is a mixture formed when a solid, liquid or gaseous substance is homogeneously mixed with a liquid. Likewise, a solvent is a substance in which another substance dissolves. To learn more about Properties, Types, Videos & Examples of Solution Visit BYJU'S. Gas Laws: Boyle's Law, Charle's Law, Gay-Lussac's Law ... Here, n is the number of moles of the gas. Hence, V= k 4 n. The number of molecules in a mole of any gas is known as the Avogadro’s constant and is calculated to be 6.022 * 10 23. The values for temperature and pressure here are the standard values. For temperature, we take it to be 273.15 K while for the pressure it equals 1 bar or 10 5 ... Fundamentals of Analytical Chemistry- 9th Edition - Academia.edu Polyfunctional acids and bases play important roles in many chemical and biological systems. The human body contains a complicated system of buffers within cells and within bodily fluids, such as human blood.
































0 Response to "43 the mole and avogadro's number worksheet"
Post a Comment